Coordination Compounds
Maharashtra Board-Class-12-Chemistry-Chapter-9
Solutions
Question 1. Choose the most correct option.
(i) The oxidation state of cobalt ion in the complex [Co(NH3)5Br]SO4 is
(a) +2
(b) +3
(c) +1
(d) +4
(b) +3
Explanation:
We know, sulphate ion has a charge −2 i.e., SO42−
Then, [Co(NH3)5Br]2+
The oxidation state of NH3 = 0
The oxidation state of Br = −1
Let the oxidation state of Cobalt ion is x
Then, x + (3 x 0) x 5 + (−1) = +2
x – 1 = +2
x = +3
Therefore the oxidation state of cobalt ion is +3.
(ii) IUPAC name of the complex [Pt(en)2(SCN)2]2+ is
(a) bis (ethylenediamine) dithiocyanatoplatinum (IV) ion
(b) bis (ethylenediamine) dithiocyantoplatinate (IV) ion
(c) dicyanatobis (ethylenediamine) platinate IV ion
(d) bis (ethylenediammine)dithiocynato platinate (IV) ion
(a) bis (ethylenediamine) dithiocyanatoplatinum (IV) ion
(iii) Formula for the compound sodium hexacynoferrate (III) is
(a) [NaFe(CN)6]
(b) Na2[Fe(CN)6]
(c) Na[Fe(CN)6]
(d) Na3[Fe(CN)6]
(d) Na3[Fe(CN)6]
(iv) Which of the following complexes exist as cis and trans isomers ?
(1) [Cr(NH3)2Cl4]− (2) [Co(NH3)5Br]2+
(3) [PtCl2Br2]2− (square planar) (4) [FeCl2(NCS)2] 2− (tetrahedral)
(a) 1 and 3
(b) 2 and 3
(c) 1 and 3
(d) 4 only
(a) 1 and 3
(v) Which of the following complexes are chiral ?
(1) [Co(en)2Cl2]+ (2) [Pt(en)Cl2] (3) [Cr(C2O4)3]3− (4) [Co(NH3)4Cl2] +
(a) 1 and 3
(b) 2 and 3
(c) 1 and 4
(d) 2 and 4
(a) 1 and 3
(vi) On the basis of CFT predict the number of unpaired electrons in [CrF6]3−.
(a) 1
(b) 2
(c) 3
(d) 4
(c) 3
Explanation:
- The [CrF6]3− complex contains a chromium ion with a +3 oxidation state. In this state, chromium has an electron configuration of
- 3d3, meaning there are three electrons in the d orbitals.
- According to Crystal Field Theory for an octahedral complex, the d−orbitals split into two sets: t2g (lower energy) and eg (higher energy). Fluorine ligands are considered weak field ligands, and they cause a small energy gap between the t2g and eg
- A chromium ion with three d−electrons will fill the three t2g orbitals with one electron each to minimize repulsion, according to Hund's rule. As a result, all three d−electrons are unpaired.
- Therefore, the [CrF6]3− complex has three unpaired electrons.
(vii) When an excess of AgNO3 is added to the complex one mole of AgCl is precipitated. The formula of the complex is
(a) [CoCl2(NH3)4]Cl
(b) [CoCl(NH3)4] Cl2
(c) [CoCl3(NH3) 3]
(d) [Co(NH3)4]Cl3
(a) [CoCl2(NH3)4]Cl
(viii) The sum of coordination number and oxidation number of M in [M(en)2C2O4]Cl is
(a) 6
(b) 7
(c) 9
(d) 8
(c) 9
Question 2. Answer the following in one or two sentences.
(i) Write the formula for tetraammineplatinum (II) chloride.
Formula of tetraamineplatinum(II) chloride : [Pt(NH3)4]Cl2
(ii) Predict whether the [Cr(en)2(H2O)2]3+ complex is chiral. Write structure of its enantiomer.
(a) Complex [Cr(en)2(H2O)2]3+is chiral.
(b) Structure of enantiomer.

(iv) Name the Lewis acids and bases in the complex [PtCl2(NH3)2].
Lewis acid : Platinum (II) : Pt2+
Lewis bases : Chloride ion : Cl− and ammonia : NH3
(v) What is the shape of a complex in which the coordination number of central metal ion is 4 ?
A complex with the coordination number of central metal ion equal to 4 may be tetrahedral or square planar.
(vi) Is the complex [CoF6] cationic or anionic if the oxidation state of cobalt ion is +3 ?
In the complex, Co carries +3 charge while 6F− carry −6 charge. Hence the net charge on the complex is −3. Therefore it is an anionic complex.
(vii) Consider the complexes [Cu(NH3)4][PtCl4] and [Pt(NH3) 4] [CuCl4]. What type of isomerism these two complexes exhibit?
Since in these two given complexes, there is an exchange of ligands between cationic and anionic constituents, they exhibit coordination isomerism.
(viii) Mention two applications of coordination compounds.
(1) In biology :
- Several biologically important natural compounds are metal complexes. They play important role in a number of processes occuring in plants and animals.
- For example, chlorophyll present in plants is a complex of Mg. Haemoglobin present in blood is a complex of iron.
(2) In medicines :
- Pt complex cisplatin is used in the treatment of cancer.
- EDTA is used for treatment of lead poisoning.
Question 3. Answer in brief.
(i) What are bidentate ligands ? Give one example.
Bidentate ligand : This ligand has two donor atoms in the molecule or ion.
- For example, ethylenediamine, H2N−(CH2)2−NH2.

(ii) What is the coordination number and oxidation state of metal ion in the complex [Pt(NH3)Cl5] ?
In the complex, for pt, Coordination number = 6 and Oxidation state = + 4.
(iii) What is difference between a double salt and a complex ? Give an example.
| Double salt | Complex |
| Double salts exist only in the solid state and dissociate into their constituent ions in the aqueous solutions. | Coordination compounds exist in the solid−state as well as in the aqueous or non−aqueous solutions. |
| Double salts lose their identity in the solution. | They do not lose their identity completely. |
| The properties of double salts are same as those of their constituents. | The properties of coordination compounds are different from their constituents. |
| Metal ions in the double salts show their normal valence. | Metal ions in the coordination compounds show two valences namely primary valence and secondary valence satisfied by anions or neutral molecules called ligands. |
| For example in K2SO4.Al2(SO4)3.24H2O. The ions K+, Al3+ and SO4 show their properties. | In K4[Fe(CN)6], ions K+ and [Fe(CN)6]4− ions show their properties. |
(iv) Classify following complexes as homoleptic and heteroleptic
(a) [Cu(NH3)4]SO4, (b) [Cu(en)2(H2O)Cl]2+, (c) [Fe(H2O)5(NCS)]2+, (d) tetraammine zinc (II) nitrate.
Homoleptic complex :
(a) [Cu(NH3)4]SO4 (d) Tetraaminezinc(II) nitrate : [Zn(NH3)4](NO3)2
Heteroleptic Complex :
(b) [Cu(en)2(H2O)Cl]2+, (c) [Fe(H2O)5(NCS)]2+,
(v) Write formulae of the following complexes
(a) Potassium amminetrichloroplatinate (II)
(b) Dicyanoaurate (I) ion
(a) Potassium amminetrichloroplatinate(II) : K[Pt(NH3)Cl3]
(b) Dicyanoaurate(I) ion : [Au(CN)2]−
(vi) What are ionization isomers ? Give an example.
Isomers that involve the exchange of ligands between coordination and ionization spheres are called ionization isomers.
e.g. [Co(NH3)5SO4]Br and [Co(NH3)5Br]SO4
(vii) What are the high−spin and low−spin complexes ?
High spin and low spin Complexes :
(1) High spin complex (HS) :
- The complex which has greater number of unpaired electrons and hence higher value of resultant spin and magnetic moment is called high spin (or spin free) or HS complex.
- It is formed with weak field ligands and the complexes have lower values for crystal field splitting energy (CFSE), Δ0.
- The paramagnetism of HS complex is larger.
(2) Low spin complex (LS) :
- The complex which has the least number of unpaired electrons or all electrons paired and hence the lowest (or no) resultant spin or magnetic moment is called low spin (or spin paired) or LS complex.
- It is formed with strong field ligands and the complexes have higher values of crystal field splitting energy (Δ0).
- Low spin complex is diamagnetic or has low paramagnetism.
(viii) [CoCl4]2− is tetrahedral complex. Draw its box orbital diagram. State which orbitals participate in hybridization.
27Co [Ar] 3d7 4s2
Oxidation state of Co = +2
Co2+ [Ar] 3d7 4s0
Since Cl− is a weak ligand, there is no pairing of electrons. Since C.N. is 4, there is sp3−hybridisation.

(ix) What are strong field and weak field ligands ? Give one example of each.
When the ligands approach the metal atom or ion for the formation of a complex, they influence the valence electrons of metal atom or ion. Accordingly the ligands are classified as (A) strong ligands and (B) weak ligands.
Strong ligands :
They cause the pairing of unpaired electrons present in the metal atom or ion.
Strong field ligands are those in which donor atoms are C, N or P. Thus CN—, NC—, CO, HN3, EDTA, en (ethylenediammine) are considered to be strong ligands.
They cause larger splitting of d orbitals and pairing of electrons is favoured. These ligands tend to form low spin complexes.
Weak ligands :
- The weak ligands have no effect on the electrons in the valence shell of a metal atom or ion.
- Weak field ligands are those in which donor atoms are halogens, oxygen or sulphur. For example,
- F—, Cl—, Br—, l—, SCN—, C2O42—. In case of these ligands the Δ0 parameter is smaller compared to the energy required for the pairing of electrons, which is called as electron pairing energy.
The ligands then can be arranged in order of their increasing field strength as
I− < Br− < Cl− < S2− < F− < OH− < C2O42− < H2O < NCS− < EDTA < NH3 < en < CN− < CO.
(x) With the help of crystal field energy level diagram explain why the complex [Cr(en)3]3+ is coloured ?
Cr+3 = [Ar] 3d34s°
Since (en) is a strong field ligand there is pairing of electrons. The electrons occupy the t2g orbitals of lower energy.
It has one unpaired electron. Due to d−d transition, it is coloured.

Question 4. Answer the following questions.
(i) Give valence bond description for the bonding in the complex [VCl4]−. Draw box diagrams for free metal ion. Which hybrid orbitals are used by the metal ? State the number of unpaired electrons.
Since Cl− is a weak ligand, there is no pairing of electrons.
Number of unpaired electrons = 2
Type of hybridisation = sp3
Geometry of complex ion = Tetrahedral
The complex ion is paramagnetic.

(ii) Draw qualitatively energy−level diagram showing d−orbital splitting in the octahedral environment. Predict the number of unpaired electrons in the complex [Fe(CN)6]4−. Is the complex diamagnetic or paramagnetic? Is it coloured? Explain.
(a) d−orbital splitting in the octahedral environment :
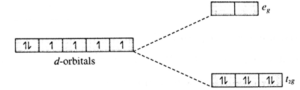
(b) [Fe(CN)6]4− is an octahedral complex.
(c) Since CN− is a strong ligand, there is pairing of electrons and the complex is diamagnetic.
(d) The complex exists as lemon yellow crystals.
(In the complex all electrons in t2g are paired and requires high radiation energy for excitation.)
(iii) Draw isomers in each of the following
(a) Pt(NH3)2ClNO2
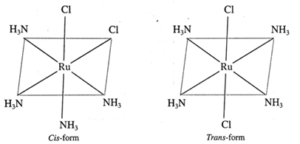
(b) Ru(NH3)4Cl2
(c) [Cr(en2)Br2]+
(a) Pt(NH3)2ClNO2
Geometric isomers :
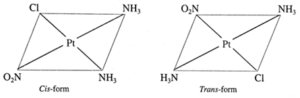
(b) Ru(NH3)4Cl2
Geometric isomers :
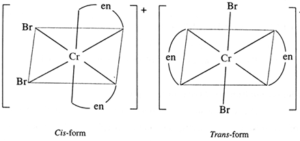
(c) [Cr(en2)Br2]+
Optical isomers :
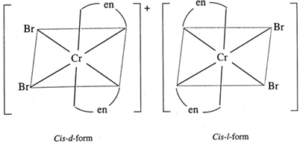
Geometric isomers :
(iv) Draw geometric isomers and enantiomers of the following complexes.
(a) [Pt(en)3]4+
(b) [Pt(en2)ClBr]2+
(a) The complex [Pt(en)3]4+ has two optical isomers.

(b) Optical isomers of [Pt(en2)ClBr]2+

(v) What are ligands ? What are their types ? Give one example of each type.
(a) In the coordination compound, the species surrounding the central metal atom or ion are called ligands or donor groups.
For example in [Cu(CN)4]2−, four CN− ions are ligands coordinated to central metal ion Cu2+.
(b) Ligands can be classified on the basis of number of electron donor atoms in the ligand i.e. denticity.
(1) Monodentate or unidentate ligand : A ligand molecule or an ion which has only one donor atom with a lone pair of electrons forming only one coordinate bond with metal atom or ion in the complex is called monodentate or unidentate ligand. For example NH3, Cl−, OH−, H2O, etc.
(2) Polydentate or multidentate ligand : A ligand molecule or an ion which has two or more donor atoms with the lone pairs of electrons forming two or more coordinate bonds with the central metal atom or ion in the complex is called polydentate or multidentate ligand. For example, ethylene diamine, H2N−(CH2)2−NH2.
Based on the number of donor atoms, polydentate ligands are further classified as: :
(i) Bidentate ligand : This ligand has two donor atoms in the molecule or ion.
- For example, ethylenediamine, H2N−(CH2)2−NH2.

(ii) Tridentate ligand : This ligand molecule has three donor atoms or three sites of attachment.
- g. Diethelene triamine, H2N−CH2−CH2−NH−CH2−CH2−NH2. This has three N donor atoms.
(iii) Tetradentate (or quadridentate) ligand : This ligand molecule has four donor atoms.
- g. Triethylene tetraamine which has four N donor atoms.
(iv) Hexadentate ligands: The ligands which bind to central metal through six donor atoms are called hexadentate ligands.
e.g. Ethylenediaminetetraacetate ion (EDTA)4− binds to metal by electron pairs of four oxygen and two nitrogen.

(3) Ambidentate ligand : A ligand molecule or an ion which has two or more donor atoms, however in the formation of a complex, only one donor atom is attached to the metal atom or an ion is called ambidentate ligand.
- Example : NO2 which has two donor atoms N and O forming a coordinate bond, M ← ONO (nitrito) or M ← NO2 (nitro).
(4) Bridging ligand : A monodentate ligand having more than one lone pairs of electrons, hence can attach to two or more metal atoms or ions and hence acts as a bridge between different metal atoms is called bridging ligand.
- Example : OH−, F−, SO42−, etc.
(vi) What are cationic, anionic and neutral complexes? Give one example of each.
On the basis of charge on complex ion, coordination complex is classified as:
(1) Cationic complex: A positively charged coordination sphere or a coordination compound having a positively charged coordination sphere is called the cationic complex or cationic sphere complex.
e.g. [Zn(NH3)4]2+
(2) Anionic complex: A negatively charged coordination sphere or a coordination compound having a negatively charged coordination sphere is called an anionic complex or anionic sphere complex.
e.g. [Zn(CN)6]3−
(3) Neutral sphere complexes: A coordination complex that does not possess a cationic or anionic sphere are neutral complexes of neutral sphere complexes.
e.g. [Ni(CO)4]
(vii) How stability of the coordination compounds can be explained in terms of equilibrium constants ?
- The stability of coordination compounds can be explained by knowing their stability constants.
- The stability is governed by metal−ligand interactions.
- In this the metal serves as electron−pair acceptor while the ligand as Lewis base (since it is electron donor).
- The metal−ligand interaction can be realized as the Lewis acid−Lewis base interaction. Stronger the interaction greater is stability of the complex.
Consider the equilibrium for the metal ligand interaction :
Ma+ + nLx— ⇌ [MLn](a+)+(nx—)
where a, x, [a+ + nx− ] denote the charge on the metal, ligand and the complex, respectively.
Now, the equilibrium constant K is given by
K = \(\frac{[ML_n]^{a+ + nx−}}{[m^{a+}][L^{x−}]_n}\)
Stability of the complex can be explained in terms of K. Higher the value of K larger is the thermodynamic stability of the complex.
The equilibria for the complex formation with the corresponding K values are given below.
Ag+ + 2CN ⇌ [Ag(CN)2] — K = 5.5 ×1018
Cu2+ + 4CN— ⇌ [Cu(CN)4]2— K = 2.0 ×1027
Co3+ + 6NH3 ⇌ [Co(NH3)6]3+ K = 5.0 ×1033
From the above data, [Co(NH3)6]3+ is more stable than [Ag(CN)2] — and [Cu(CN)4]2—.
(viii) Name the factors governing the equilibrium constants of the coordination compounds.
Factors which govern stability of the complex : Stability of a complex is governed by (a) charge to size ratio of the metal ion and (b) nature of the ligand.
- Charge to size ratio of the metal ion : Higher the ratio greater is the stability. For the divalent metal ion complexes their stability shows the trend : Cu2+ > Ni2+ > Co2+ > F2+ > Mn2+ > Cd2+ . The above stability order is called Irving−William order. In the above list both Cu and Cd have the charge + 2, however, the ionic radius of Cu2+ is 69 pm and that of Cd2+ is 97 pm. The charge to size ratio of Cu2+ is greater than that of Cd2+. Therefore the Cu2+ forms stable complexes than Cd2+.
- Nature of the ligand : A second factor that governs stability of the complexes is related to how easily the ligand can donate its lone pair of electrons to the central metal ion that is, the basicity of the ligand. The ligands those are stronger bases tend to form more stable complexes.
PDF : Chapter-9-Coordination Compounds-Text Book
PDF : Chapter-9-Coordination Compounds- Notes
PDF : Chapter-9-Coordination Compounds- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 8- Transition and Inner transition Elements – Online Solutions
Next Chapter : Chapter-10-Halogen Derivatives– Online Solutions
We reply to valid query.