Adsorption and Colloids
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -11
Notes-Part-2
Topics to be Learn : Part-2
|
Colloids :
- Colloids are found in a variety of everyday items. Milk, butter, jelly, whipped cream, and mayonnaise are a few examples.
- The usage of colloids is significant in many daily operations such as cooking, washing, dyeing, painting, ore floatation, water purification, sewage disposal, smoke prevention, photography, and medicine.
Colloidal state : The colloidal state of a substance refers to a particular particle size of the substance, ranging between 2nm to 500 nm which it acquires on treatment with a solvent.
Colloidal dispersion or colloids : Colloidal dispersion (or colloids) is a heterogeneous two phase system, with the dispersion medium as one phase and the dispersed substance as the other phase and there is a definite surface of separation between each colloidal particle and the dispersion medium.
Dispersed phase : The colloidal particles of the size ranging between 2 nm to 500 nm that are present in the dispersion medium represent the disperse phase which is a discontinuous phase.
Dispersion medium : The medium in which the disperse phase of colloidal particles exist is called a dispersion medium and it is a continuous phase.
True solution : A homogeneous transparent solution is called a true solution and the particle size is in the range of 0.1 to 2 nm. For example, aqueous solution of NaCl, sugar, etc.
Colloidal solution or a colloidal dispersion : A solution which constitutes a dispersed phase of colloidal particles of the size 2 nm to 500 nm and the dispersion medium is called a colloidal solution or colloidal dispersion or colloidal system or disperse system.
Sol : A dispersion of a solid in a liquid dispersion medium or of a solid in a solid dispersion medium is called a sol.
Aerosol : A dispersion of a solid or a liquid in a gas as a dispersion medium is called aerosol. e.g. smoke.
Examples of colloids :
Examples of phenomenon observed in our daily life in terms of colloids are as follows :
- Blue colour of the sky : The sky appears blue to us because minute dust particles along with minute water droplets dispersed in air scatter blue light which reaches our eyes.
- Blood : It is a colloidal dispersion of plasma proteins and antibodies in water. (At the same time blood is also a suspension of blood cells and platelets in water.)
- Soils : Fertile soils are colloidal in nature where humus acts as a protective colloid. Soil adsorbs moisture and nourishing materials due to its colloidal nature.
- Fog, mist and rain : Mist is caused by small droplets of water dispersed in air. Fog is formed whenever there is temperature difference between ground and air. A large portion of air containing dust particles gets cooled below its dew point, the moisture from the air condenses on the surface of these particles which form fine droplets, which are colloid particles and float in air as fog or mist.
Classification of colloids :
Colloids are classified in three different ways.
(i) Classification of colloids based on physical state : Below Table illustrates the types of colloids in accordance with the physical states of dispersed phase and dispersion medium.
Types of colloids based on physical state :
| Dispersed phase | Dispersion medium | Type of colloid | Examples |
| solid | solid | solid sol | coloured glasses, gem stones, porcelain, paper |
| solid | liquid | sols and gels | paints, cell fluids, gelatin, muddy water, starch solution. |
| solid | gas | aerosol | smoke, dust |
| liquid | solid | gel | cheese, butter, jellies |
| liquid | liquid | emulsion | milk, hair cream |
| liquid | gas | aerosol | fog, mist, cloud, hair sprays, insecticide sprays. |
| gas | solid | solid sol | pumice stone, foam rubber, plaster |
| gas | liquid | foam | froth, whipped cream, soap lather |
Classification of colloids based on interaction or affinity of phases : On the
basis of interaction or affinity of phases, a colloidal solution is classified as lyophilic and lyophobic.
- Lyophilic colloids : The sols in which there is a strong affinity betweens the dispersed phase and the dispersion medium are called lyophilic colloids.
- Lyophobic colloids : The sols in which there is little affinity between the dispersed phase and the dispersion medium are called lyophobic colloids.
Distinction between Lyophilic and Lyophobic colloids :
| Lyophilic colloids | Lyophobic colloids |
| 1. Formed easily by direct mixing. | Formed only by special methods. |
| 2. Reversible. | Irreversible. |
| 3. The particles are not easily visible even under ultramicroscope. | The particles are easily detected under ultramicroscope. |
| 4. These are self stabilized. | These are unstable and hence require traces of stabilizers. |
| 5. Addition of large amount of electrolytes causes precipitation /coagulation. | Addition of small amount of electrolytes causes precipitation/ coagulation. |
| 6. Viscosity of dispelrsed phase much higher than that of the dispersion medium. | Viscosity of dispersed phase is nearly the same as the dispersion medium. |
| 7. Surface tension of dispersed phase is lower than dispersion medium. | Surface tension of dispersed phase is nearly the same as the dispersion medium. |
Classification of colloids based on molecular size :
Colloids are classified into three types in accordance with size of their molecules.
- Multimolecular colloids
- Macromolecular colloids
- Associated colloids or micelles
Multimolecular colloids :
- Multimolecular colloids are the systems in which the disperse phase particles (colloidal particles) are aggregates of many atoms or molecules of the size less than 103 pm and held together by van der Waal forces.
- For example, Gold sol, Ag sol, sulphur sol.
Macromolecular colloids :
- Macromolecular colloids are the systems in which the colloidal or disperse phase particles are not aggregate of molecules but are single molecules of colloidal size (1 nm to 103 nm).
- For example, starch, cellulose, proteins, polythene, nylon, plastics, etc.
Associated colloids or micelles :
- Many substances like sodium salt of long size fatty acids behave as electrolytes in aqueous solution.
- As their concentration increases they get associate forming a colloidal solution.
- The associated particles are called micelles.
For example, soap, detergents.
Micelle formation in a soap solution :

- Soap has a long hydrophobic hydrocarbon chain, called tail, attached to hydrophilic ionic group carboxylate, called head.
- In water, the soap molecules arrange themselves to form spherical particles that are called micelles. See above Fig.
- In each micelle, the hydrophobic tails of soap molecules point to the centre and the hydrophilic heads lie on the surface of the sphere.
- As a result of this, soap dispersion in water becomes stable.
Preparation of Colloids :
A few important methods for the preparation of colloids are as follows :
(i) Chemical methods : By double decomposition, oxidation, reduction or hydrolysis. Molecules of water insoluble products of these reaction aggregate together and form colloids or sols.
Examples :
SO2 + 2H2S \(\underrightarrow{Oxidation}\) 3S ↓ + 2H2O
2AuCl3 + 3HCHO + 3H2O \(\underrightarrow{Reduction}\) 2Au ↓ +3HCOOH + 6HCl
FeCl3 + 3H2O \(\underrightarrow{Hydrolysis}\) Fe(OH)3 ↓ + 3HCl
(ii) Electrical disintegration by Bredig’s Arc method :
- This process involves vaporization as well as condenstion.
- In this method, electric arc is struck between electrodes of metal immersed in the dispersion medium.
- The intense heat produced vapourises the metal which then condenses to form particles of colloidal sol.

- Colloidal sols of metals such as gold, silver, platinum can be prepared by this method.
(iii) Peptization :
- It is redespersion of a precipitate into a colloidal sol by shaking with dispersion medium in the presence of a small amount of an electrolyte.
- The electrolyte is called peptizing agent, which has a common ion.
- For example, a precipitate of CuS is peptized by H2
Purification of colloidal solution :
- The process used for reducing the amount of impurities to a requisite minimum is known as purification of colloidal solution.
- Colloidal solution generally contains excessive amount of electrolytes and some other soluble impurities. A small quantity of an electrolyte is necessary for the stability of colloidal solution. But a large quantity of electrolyte may result in coagulation. It is also necessary to reduce soluble impurities.
- Purification of colloidal solution can be carried out using dialysis
Dialysis :
It is a process of removing a dissolved substance from a colloidal solution by diffusion through a suitable membrane. Dialysis is carried by a dialyser.

A bag of suitable membrane containing the colloidal solution is suspended in a vessel through which fresh water is continuously flowing. The molecules and ions diffuse through membrane into the outer water while pure colloidal solution is left behind.
Properties of colloidal dispersions :
Various properties exhibited by colloidal dispersions are as following :
General properties :
- Colloidal system is heterogeneous and consists of two phases, dispersed phase and dispersion medium.
- The dispersed phase particles pass slowly through parchment paper or animal
- membrane, but readily pass through ordinary filter paper.
- The particles usually are not detectable by powerful microscope.
The colloidal systems exhibit optical property like Tyndal effect, kinetic property like Brownian motion, electrical properties like electrophoresis.
Optical property:
Tyndall observed that when light passes through true solution the path of light through it cannot be detected.
Tyndall effect : The phenomenon of scattering of light by colloidal particles and making path of light visible through the dispersion is referred as Tyndall effect.

- When a light passes through a true solution, containing solute particles of size less than 1 nm, the path of the light is not visible.
- If the light is passed through a colloidal solution, the path of the light through the dispersion medium becomes visible due to the scattering of the light. This is the optical property of the colloids.
- The bright cone of the light observed is called Tyndall cone.
Conditions for Tyndall effect :
- The diameter of the dispersed particles is not much smaller than the wavelength of light used.
- The refractive indices of dispersed phase and dispersion medium differ largely.
Importance of Tyndall effect :
- It is useful in determining number of particles in colloidal system and the particle size therein.
- It is used to distinguish between colloidal dispersion and true solution.
Colour property:
- Colour of colloidal solution depends on the wavelength of light scattered by dispersed particles.
- The colour of colloidal dispersion also changes with the manner in which the observer receives the light. For example: Mixture of a few drops of milk and large amount of water appears blue when viewed by the scattered light and red when viewed by transmitted light.
- It also depends on size of colloidol particles For example, finest gold sol is red in colour whereas with increase in size it appears purple.
Kinetic property of colloids :
Brownian motion : The random ceaseless motion of the colloidal or disperse phase particles taking place in all directions over a largo area is called Brownian motion.
Cause of Brownian motion :
- The colloidal particles are constantly being bombarded by the molecules of the dispersion medium, which causes this movement.
- Due to collisions with the molecules in the dispersion medium, the colloidal particles gain kinetic energy.
- The Brownian motion of the colloidal particles is substantially slower than that of the medium molecules because they are heavier than the molecules of the dispersion medium.

Electrical Properties :
(i) Charge on colloidal particles: Colloidal particles carry an electric charge. The nature of this charge is the same on all particles for a given colloidal solution which can be either positive or negative. Some common sols with the nature of charge on the particles are listed in below Table.
| Positively charged sols | Negatively charged sols |
| 1. Hydrated metallic oxides Al2O3.xH2O, CrO3.xH2O, Fe2O3.xH2O. | Metals, Cu, Ag, Au Sols metallic sulphides As2S3, Sb2S3, Cds |
| 2. Basic dye stuff, methylene blue sols | Acid dye stuff, eosin, congo red sol |
| 3. Haemoglobin (blood) | Sols of starch, gum |
| 4. Oxides : TiO2 sol | Gelatin, clay, gum sols |
(ii) Electrophoresis : The movement of colloidal particles under an applied electric potential is called electrophoresis.
Electrophoresis set up :
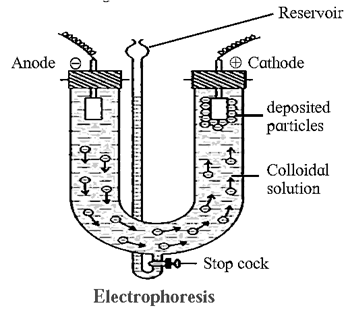
- Above figure shows U tube set up in which two platinum electrodes are dipped in a colloidal solution.
- When electric potential is applied across two electrodes, collodial particles move towards one or other electrode.
- Positively charged particles move towards cathode while negatively charged particles migrate to anode and get deposited on the respective electrode.
Applications :
- To decide the electrical charge on the colloidal particles.
- To separate colloidal particles and obtain a pure true solution.
- Since the components of colloidal particles in a mixture migrate with different rates, they can be separated.
- The rate of migration of colloidal particles can be measured.
(iii) Electroosmosis : The migration of a dispersion medium from a colloidal solution under the influence of an electric field is called electroosmosis.
In electroosmosis movement of dispersed particles is prevented by suitable means, such as use of membrane. Then it is observed that the dispersion medium begins to move in an electric field.
- In this, a U shaped tube divided in two parts by inserting a membrane at the centre is used.
- One arm is filled with a colloidal solution and another arm with dispersion medium and two electrodes are introduced in two arms.
- When electric potential is applied, dispersed phase particles remain stationary while dispersion medium from colloidal sol migrates towards the electrode with a charge opposite to charge on colloidal particles.
Coagulation : It is defined as the precipitation of colloids by removal of charge associated with the colloidal particles.
Explanation :
- A common method to coagulate a sol is the addition of an excess of an electrolyte.
- When an electrolyte is added to a sol, the charged colloidal particles adsorb the ions of the charge opposite to that present on the colloidal particles and thus their charge is neutralised.
- These neutral particles aggregate forming large size particles and coagulate.
Methods to effect coagulation :
The coagulation of a sol can be carried out by different methods as follows :
- By electrophoresis : In the electrophoresis process, the colloidal particles migrate to the oppositely charged electrodes in the presence of an applied electric field and get discharged. These particles eventually aggregate and coagulate.
- By mixing oppositely charged colloidal sols : When two sols carrying opposite charges are mixed, both the sols get mutually neutralized and coagulate. For example, addition of positively charged colloidal solution of Fe(OH)3 to an equal amount of negatively charged sol of As2S3 causes coagulation of both the sols.
- By boiling the sol : When the sol is boiled, the adsorbed ions on colloidal particles are removed and neutral particles undergo rapid collisions in dispersion medium and coagulate.
- By persistent dialysis : On persistent dialysis, the adsorbed ions on colloidal particles are removed. The colloidal particles then become unstable and finally precipitate.
- By addition of electrolytes : When an excess of electrolyte is added, the colloidal particles undergo coagulation.
Hardy-Schulze rule : The ability of the flocculating ion to precipitate is often inversely proportional to its valence. This is referred to as the Hardy-Schulze rule.
(i) Ions of the added electrolyte neutralize oppositely charged colloidal particles in the colloidal solution and cause the precipitation.
(ii) The precipitation power of an electrolyte increases with the increase in the valence of an anion or a cation of the electrolyte.
For example, Na+ < Mg+ < Al2+ and Cl— < SO42— < PO43—
- The lyophilic colloids require large amount of electrolyte for their coagulation, because the lyophilic particles are surrounded by a layer of medium through which the penetration of ions becomes more difficult.
- The higher concentration of the added electrolyte removes this layer of medium and ions neutralise the charges of colloidal particles and coagulation takes place.
- Lyophobic sols require less amounts of the electrolyte of coagulation.
Emulsions : A colloidal system in which one liquid is dispersed in another immiscible liquid is called an emulsion.
- There are liquid-liquid colloidal system in which both liquids are completely or partially immiscible.
There are two types of emulsions
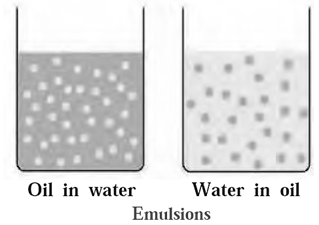
(i) Emulsion of oil in water (o/w type) : An emulsion in which dispersed phase is oil anddispersion medium is water is called emulsion of oil in water.
For example : milk, vanishing cream, paint etc. Milk consists of particles of fat dispersed in water.
(ii) Emulsion of water in oil (w/o type): An emulsion in which dispersed phase is water and dispersion medium is oil is called emulsion of water in oil.
For example, codliver oil consists of particles of water dispersed in oil. Some other examples of this type include butter, cream, etc.
Preparation :
- Emulsions are prepared by shaking vigorously a mixture of two immiscible liquids. Emulsions are lyophobic and unstable. Hence a third substance called an emulsifying agent also called emulsifier is added to the emulsion during its preparation.
- The emulsifiers are soaps, detergents, long chain sulphonic acids, lyophilic sols, alkyl sulphonates, etc.
- The emulsifier forms a protective layer surrounding the disperse phase or colloidal particle and converts lyophobic emulsion into lyophilic emulsion.
- Hence, emulsion is prepared by mixing an emulsifier with a dispersion medium and then adding the disperse phase slowly with constant stirring.
Distinction between oil in water and water in oil emulsions :
| Oil in water | Water in oil |
| 1. Oil is the dispersed phase and water is the dispersion medium. | Water is dispersed phase and oil is the dispersion medium. |
| 2. If water is added, it will be miscible with the emulsion. | If oil is added, it will be miscible with the emulsion. |
| 3. An addition of small amount of an electrolyte makes the emulsion conducting. | Addition of small amount of an electrolyte has no effect on conducting power. |
| 4. Water is continuous phase. | Oil is continuous phase. |
| 5. Basic metal sulfates, water soluble alkali metal soaps are used as emulsifiers. | Water insoluble soaps such as those of Zn, Al, Fe, alkaline earth metals are used as emulsifiers. |
Properties of Emulsion :
- Emulsion can be diluted with any amount of the dispersion medium. On the other hand, the dispersed liquid when mixed forms a separate layer.
- The droplets in emulsions are often negatively charged and can be precipitated by electrolytes.
- Emulsions show Brownian movement and Tyndall effect.
- The two liquids in emulsions can be separated by heating, freezing or centrifuging etc.
Applications of colloids : Colloids find applications in industry and in daily life.
Following are some examples.
- Electrical precipitation of smoke : Smoke is colloidal solution of solid particles of carbon, arsenic compound, dust etc. in air. When smoke is allowed to pass through chamber containing plates having charged smoke particles they lose their charge and get precipitated. The particles settle down on the floor of the chamber. The precipitator is called Cottrell precipitator.
- Purification of drinking water: Water obtained from natural sources contains colloidal impurities. By addition of alum to such water, colloidal impurities get coagulated and settled down. This makes water potable.
- Medicines: Colloidal medicines are more effective due to large surface area to volume ratio of colloidal particles and they can be easily assimilated by body. Some of the medicines are argyrol which is a silver sol used as an eye lotion, an emulsion of milk of magnesia which is used in stomach disorders.
- Rubber Industry: - Rubber is obtained by coagulation of latex.
- Cleansing action : Cleansing action of soaps and detergents
- Industrial goods : Photographic plates, films, and industrial products like paints, inks, synthetic plastics, rubber, graphite lubricants, cement etc. are colloids.
Class-11-Chemistry-Chapter-11-Adsorption and Colloids-Text Book
Class-11-Chemistry-Chapter-11-Adsorption and Colloids- Notes
Class-11-Chemistry-Chapter-11-Adsorption and Colloids-Solution
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-10-States of Matter – Online Notes
Next Chapter : Chapter-12-Chemical Equilibrium – Online Notes