Modern Periodic Table
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -7
Notes
|
Topics to be Learn :
|
Introduction :
- Around 30 elements were known in the early nineteenth century and were classified as metals, nonmetals, and metalloids based on their physical properties.
- The elements were classified according to their atomic mass in Dobereiner's triads and Newland's octaves.
Mendeleev’s periodic law : Mendeleev’s periodic law states that ”The physical and chemical properties of elements are periodic function of their atomic masses”.
- Mendeleev classified the elements using the atomic mass and their properties.
- The periodic table was based on ’Mendeleev’s periodic law.’
Salient features of Mendeleev’s periodic table :
- Mendeleev arranged the elements (known at the time) in increasing atomic mass order.
- The atomic number was the serial or ordinal number of an element in this order.
- The periodic table is divided into vertical columns known as groups and horizontal rows (now called as periods.)
- Properties in a period changed gradually from left to right, while elements with similar properties were grouped together.
- He left some gaps corresponding to certain atomic numbers in the periodic table to maintain periodicity of the properties.
- Newly discovered elements fitted into the gaps with their properties as predicted by the Mendeleev’s periodic law.
- Inert gases, discovered in later years could be accommodated in this periodic table by creating an additional group.
- The elements belonging to the same group have similar electronic configuration of the valence shell. These valence electrons are involved during a chemical reaction. Hence, they have similar chemical properties
Henry Moseley’s work (1913): Henry Moseley’s work on X—ray spectroscopic studies of many elements showed that the characteristic frequency of X-ray emitted by an element was related to atomic number, Z, of the element and not the atomic mass. Thus, atomic number was considered to be the fundamental property of atom which modified Mendeleev’s periodic law. This is called Modern periodic law.
Modern periodic law : It states that "The physical and chemical properties of elements are a periodic function of their atomic numbers.”
Structure of the Modern Periodic Table :
Long form of periodic table or The modern periodic table : Periodic table based upon the principle that the physical and chemical properties of the elements are the periodic functions of their atomic numbers is called Long form of the periodic table or extended form of periodic table or modern periodic table.
Skeletal Diagram of Long form of the periodic table :
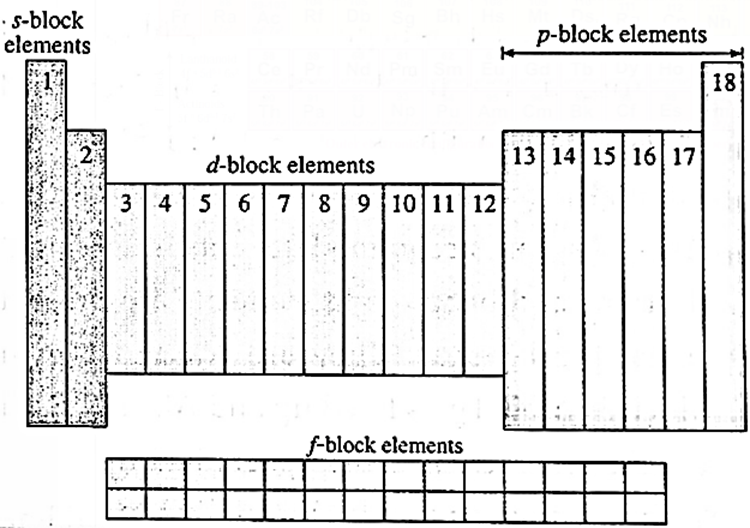
Structural features of Long form of the periodic table :
- Long form or the Modern periodic table is based on Modern periodic law. It is a revised version of Mendeleev’s periodic table.
- The modern periodic table has horizontal rows intersecting the vertical columns giving rise to a number of boxes.
- The horizontal rows are called Periods. There are seven periods numbered 1 to 7.
- The vertical columns are called Groups. There are eighteen groups numbered 1 to 18.
- The numbering of periods and groups are as per the recommendation of IUPAC, International Union of Pure and Applied Chemistry.
- There are in all 118 boxes to accommodate 118 elements in the modern periodic table.
- The modern periodic table is divided into four blocks. Two groups on the left form the s—blocks six groups on the right form the p—block, ten groups in the centre form the d—block and the two series at the bottom constitute the f-block.
- Below the main table are placed two series containing fourteen elements each. They are known as Lanthanoide series (in 6th period) and Actinoide series, (in 7th period) respectively.
There are 18 groups and according to IUPAC they are numbered from 1 to 18 :
- The elements in the same group have similar valence shell configuration and same number of electrons.
- The elements in the same group have similar properties.
- The elements of the groups 1, 2 and 13 to 17 are called normal elements.
- The elements of the groups from 3 to 12 are called transition elements.
- Two series of fourteen elements, namely lanthanoids and actinoids are placed at the bottom of the periodic table, and they are called inner transition elements. They are placed in group 3.
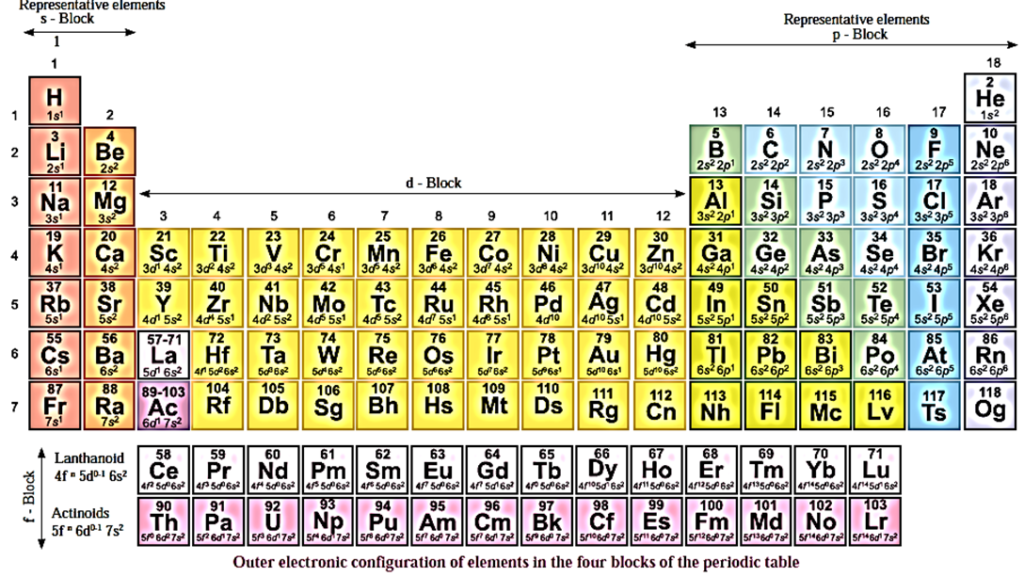
Periodic Table and Electronic configuration :
The atomic structure was unknown when Mendeleev proposed his periodic table in 1869. When arranging elements in increasing order of atomic mass, he discovered periodicity in their properties. The properties of elements were later correlated to electronic configuration with the advent of the quantum mechanical model of the atom.
Electronic configuration in periods :
There are seven periods numbered as 1 to 7.
The number of any given period corresponds to a principal shell which gets filled in an atom on moving from left to right in the period.
Along a period the atomic number increases by one and one electron is added to outermost shell. Every period ends with complete octet configuration (or duplet in the first period) of the valence shell. The next period begins with addition of electron to the next shell.
First period : This begins with filling up of the first shell, (n = 1) which has only one orbital (1s) and can accommodate maximum two electrons. Hence this period has only two elements, hydrogen (1s1) and helium (1s2).
Second period : This begins with the filling up of electrons in the second shell (n=2). The second period starts with Li (Z = 3) : 1s22s1 and ends up with Ne (Z = 10) : 1s22s22p6. Thus Ne can accommodate maximum eight electrons and hence eight elements are present in the second period. They are Li and Be in which 2s—orbital get filled up and B, C, N, O, F and Ne in which 2p—orbitals get filled up progressively.
Third period : This begins with the filling up of electrons in the third shell (n =3). The third period starts with Na (Z=11):1s22s22p63s1 and ends up with Ar (Z = 18): 1s22s22p63s23p6. Thus, Ar can accommodate maximum eight electrons and hence eight elements are present in the third period. They are Na and Mg in which 3s-orbital gets filled up and Al, Si, P, S, Cl and Ar in which 3p-orbital gets filled up.
Fourth period : This period starts with the filling up electrons in the fourth shell (n=4). After filling 4s-orbital in K (4s1) and Ca (4s2), the electrons enter higher energy 3d—orbital accommodating ten electrons and the corresponding ten elements are from Sc (Z = 21) to Zn (Z = 30), having electronic configuration from 3d14s2 to 3d104s2. After filling up of 3d—orbital, six electrons enter 4p-orbital from Ga (3d104s24p1) to Kr(3d104s24p6). Hence, fourth period consists of 18 elements (2 + 10 + 6).
Fifth period : The fifth period accommodates 18 elements as a result of successive filling of electrons in the 5s, 4d and 5p subshells. This starts with the filling up electrons in the fifth shell (n =5). In this, the electrons first enter 5s-orbital in Rb (5s1) and Sr (5s2) and then they enter 4d orbital accommodating ten electrons and corresponding ten elements are from Y(4d15s2) to Cd (4d105s2). After 4d10—orbital, six electrons enter 5p—orbital from In (4d105s25p1) to Xe (4d105s25p6). Hence,
fifth period consists of 18 elements (2+10+6).
Sixth period : This begins with the filling up electrons in the sixth shell (n = 6) in the atom. The electrons first enter 6s-orbital and ends by completing the subshell 6p. Therefore, the sixth period has the subshells filled in increasing order of energy as 6s < 4f < 5d < 6p. The electron capacities of these subshells are 2, 4, 10 and 6 respectively. Hence, the sixth period is the longest period consisting of 32 elements, (2 + 14 + 10 + 6).
Table of Orbitals, number of electrons and number of elements in each period :
| Period Number | Orbitals filled and their sequence | Electrons accommodated | Total number of elements |
| 1 | 1s | 2 | 2 |
| 2 | 2s, 2p | 2+6 | 8 |
| 3 | 3s, 3p | 2+6 | 8 |
| 4 | 4s, 3d, 4p | 2+10+6 | 18 |
| 5 | 5s, 4d, 5p | 2+10+6 | 18 |
| 6 | 6s, 4f, 5d, 6p | 2+14+10+6 | 32 |
| 7 | 7s, 5f, 6d, 7p | 2+14+10+6 | 19 out of 32 |

Electronic configuration in the four blocks:
The structure of the modern periodic table shows four blocks. These blocks are formed in accordance with the subshell in which the last electron enters.
Accordingly the four blocks are named as s-block, p-block, d-block and f-block.
s-Block elements : In the atoms of these elements, last electron enters s-orbital of the valence shell. They have electronic configuration ns1 and ns2 and they belong to group 1 and group 2 respectively. They are placed on the extreme left of the periodic table.
p-Block elements : In the atoms of these elements, the last electron enters p-orbital of the valence shell. There being three degenerate p-orbitals in a p-subshell upto 6 electrons can be filled. They have electronic configuration ns2 np1 to ns2 np6 and they belong to groups 13 to 18, on extreme right of periodic table. The p-block ends with the group 18 which represents the family of inert gases.
d-Block elements : In the atoms of these elements, last electron enters d-orbital of penultimate shell, i.e., (n—1)d-orbital. There being five orbitals in a d-subshell, 10 electrons can be accommodated. They have electronic configuration ns1-2 (n — 1)d1 to ns1-2 (n — 1)d1°, and they belong to groups 3 to 12 and they are placed in the middle portion of the periodic table. There are four d series elements namely 3d, 4d, 5d and 6d series.
f-Block elements : In the atoms of these elements, last electron enters into f-orbital of pre—penultimate shell, i.e., (n — 2) f-orbital. They have general outer electronic configuration ns2(n — 1)d0-1 (n — 2)f1-14. The f-block consists of two series of 14 elements called Lanthanides and Actinides. They are placed at the bottom of the periodic table.
Examples of some elements with period, group and block in the periodic table which they belong :
(i) 2He : 1s2
Here, n = 1. Therefore, 2He belongs to the 1st period. The shell n = 1 has only one subshell, namely 1s. The outer electronic configuration 1s2 of ’He’ corresponds to the maximum capacity of 1s, the complete duplet. Therefore, He is placed at the end of the 1st period in the group 18 of inert gases, so ’He’ belongs to p-block
(ii) 54Xe : 5s25p6
Here n = 5. Therefore, 54Xe belongs to the 5th period. The outer electronic configuration 5s25p6 corresponds to complete octet. Therefore 54Xe is placed in group 18 and belongs to p-block.
(iii) 16S : 3s23p4
Here n = 3. Therefore, 16S belongs to the 3rd period. The 3p subshell in ‘S’ is partially filled and short of completion of octet by two electrons. Therefore ‘S’ belongs to (18-2) = 16th group and p – block.
(iv) 79Au : 6s15d10
Here n = 6. Therefore, ‘Au’ belongs to the 6th period. The sixth period begins with filling of electron into 6s and then into 5d orbital. The outer configuration of ‘Au’ :
6s1 5d10 implies that (1+10) = 11 electrons are filled in the outer orbitals to give ‘Au’. Therefore ‘Au’ belongs to the group 11. As the last electron has entered ‘d’ orbital ‘Au’ belongs to the d-block.
(v) Na(z=11) : Atomic number of Na is 11.
Electronic configuration is 1s2 2s2 2p6 3s1.
- Block : It belongs to s-Block since the last (differentiating) electron enters into 3s-orbital.
- Period : It belongs to 3rd period since the principal quantum number of valence shell is 3.
- Group : It belongs to group 1 because it is in s-block and contains 1 electron in the valence shell.
Blockwise characteristics of elements
The elemental properties are characteristic of the block they belong.
Characteristics of s-block elements :
- The s-block contains elements of group 1 (alkali metals) and group 2 (alkaline earth metals).
- They are reactive metals that only exist in their combined state.
- Their compounds are mostly ionic (exception of Li and Be).
- They have low ionisation enthalpy, which decreases down the group and results in increased reactivity.
- They are called representative elements.
Characteristics of p-block elements :
- The p-block contains elements of group 13 to 18.
- Group 18 is the family of inert (noble) gases.
- Group 17 (halogens) and group 16 (chalcogens) include reactive nonmetals.
- The p-block contains all the three types of elements, metals on the left, nonmetals on the right and metalloids along a zig—zag line.
- Nonmetallic character increases from left to right, whereas it decreases down a group.
Characteristics of d-block elements :
- The d-block contains elements of group 3 to 12.
- They are all metals, known as transition elements or transition metals.
- Most of the d—block elements have a partially filled inner d-orbitals. As a result they have variable oxidation state, paramagnetism and ability to form coloured ions.
- They are used as catalysts.
- Zn, Cd and Hg have completely filled s and d subshells and hence do not show typical properties of transition elements.
Characteristics of f-block elements :
- The f-block contains elements which are all metals and are placed in two rows called lanthanoid series (58Ce to 71Lu) and actinoide series (90Th to 103Lr).
- These series are named after their preceding elements lanthanum (57La) and actinium (89Ac) in the third group of the d-block of the 6th and 7th period respectively.
- Lanthanides are also known as rare earth elements.
- As the last electron of these elements is filled in the (n-2) f subshell, these elements are called inner-transition elements.
- Actinide elements beyond 92U are called trans-uranium elements and they are radioactive.
- The f—block elements are placed separately of the bottom of the periodic table.
Periodic trends in elemental properties :
Screening effect : The inner shell electrons in an atom screen or shield the outermost valence electrons from the nuclear attraction. This effect is called screening effect or shielding effect. The magnitude of screening effect depends upon the number of inner electrons. Higher the number of inner electrons, greater is the value of screening effect. The screening constant is represented by 'σ’ (sigma).
- Example : Across a period the electrons enter the same shell. Thus, shielding due to inner electrons remain same.
- Down a group, a new larger valence shell is added which increases the inner electrons and thus the shielding effect increases.
Effective nuclear charge : Due to screening effect the valency electrons experience less attraction towards nucleus. This reduces the active nuclear charge (z) actually present on the nucleus. This reduced nuclear charge is termed as ‘effective nuclear charge. (zeff).
Effective nuclear charge (zeff) = (z) − (electron shielding) = z − σ
- Effective nuclear charge increases across a period and decreases down a group. This is because, as we move down a group, a new larger valence shell is added. As a result, there is an additional shell in the core.
Periodic trends in physical properties :
Atomic radius : Atomic radius is one half of the inter nuclear distance between two adjacent atoms of a metal or two single bonded atoms of a nonmetal.
- Atomic radius is estimated in terms of electron density surface which encloses 95% (or more) of the electron density.
Pattern of atomic size variation in a group and across a period :
In a period :
- Atomic radius decreases in a period from left to right (upto group 17).
- This is because as we move across a period, atomic number increases from left to right, nuclear charge increases but electrons enter the same shell.
- Thus, the screening effect of the inner core remains the same, on the other hand the effective nuclear charge goes on increasing.
- As a result the valence electrons are tightly held by the nucleus and atomic size (radius) decreases.
- Example : In 2nd Period, it decreases from Li(152 pm) to F(64 pm).
- Noble gases (last element in each period) are inert elements, as their outermost shell is completely filled and the octet is completed. Thus, noble gases (group 18) have larger atomic radii than preceding halogens (group 17).
In a group :
- Down the group, as the atomic number increase, the effective nuclear charge increases and shielding effect increases down a group.
- The valence electrons are held by weaker attractive forces, thus, atomic radius increases.
- Example: Atomic radius increases. For group 17 elements, the value increase from 1st member F (64 pm) to the last member At (140 pm).
Covalent radius of the atom : In the case of nonmetals (except noble gases), the atoms of an element are bonded to each other by covalent bonds. Bond length of a single bond is taken as sum of radii of the two single bonded atoms. This is called covalent radius of the atom.
Example :
- Bond length of Be-Be bond in Berylium is 224 pm. Therefore, atomic radius of Berylium is estimated to be 112pm
- Bond length of Cl-Cl bond in Cl2 is measured as 198 pm. Therefore, the atomic radius of Cl is estimated to be 99 pm.
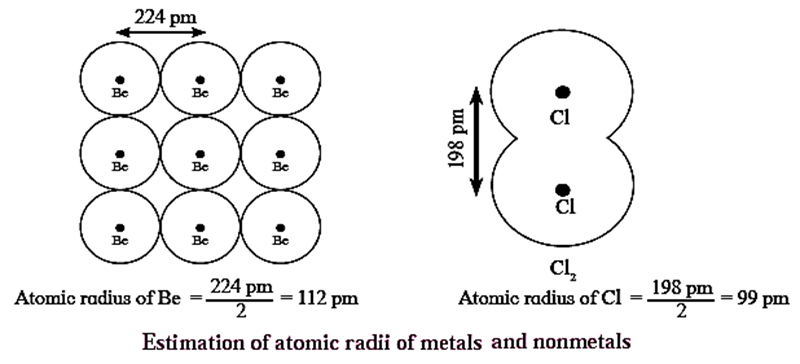
Ionic radius : Ionic radius is the distance between neighbouring cations and anions in ionic crystals.
A cation has small radius than its parent atom :
Explanation :
- A cation is formed by the loss of electron from its parent atom.
M —> M+ + e-
- The nuclear charge in a parent atom and its cation is same.
- Hence, outermost electrons in cation experience greater force of attraction i.e. larger effective nuclear charge and lesser shielding effect.
- Therefore, the radius of cation is less than its parent atom.
- For example, Na atom has atomic radius 186 pm while its cation Na+ has ionic radius 95 pm.
An anion has larger radius than its parent atom :
Explanation :
- An anion is formed by the acceptance of an electron by a parent atom.
A + e− —> A−
- The nuclear charge in a parent atom and its anion is same.
- The additional electron in an anion, results in the repulsion among the electrons and results in the decrease in effective nuclear charge, as compared to the parent atom.
- Therefore, the radius of an anion is larger than its parent atom. For example, F atom has atomic radius 64 pm while anion F has ionic radius 136 pm.
Isoelectronic species : The isoelectronic species (atoms or ions) are those which have the same number of electrons.
- Example : Na+, Mg+, Al+ have same number of electrons equal to ten, hence they are isoelectronic.
Size of isoelectronic species decreases with the increase in atomic number :
Explanation :
- Isoelectronic species have same number of electrons and same configuration.
- As atomic number increases, nuclear charge increases.
- The increasing nuclear charge acts on the same number of electrons in isoelectronic species.
- Hence, due to greater nuclear attraction, the size of isoelectronic species decreases as the atomic number increases.
Examples :
Al3+, Mg2+, Si4+, Na+ :
- All the given species, namely Al3+, Mg2+, Si4+ and Na+ are isoelectronic, having 10 electrons each.
- Their atomic numbers are Na+(11), Mg2+(12), Al3+(13) and Si4+(14).
- Hence the increasing order of nuclear attraction is Na+< Mg2+ < Al3+ < Si4+.
- Therefore ionic size decreases as follows, Na+ > Mg2+ > Al3+ > Si4+.
Ionization enthalpy : The energy required to remove an electron from the isolated gaseous atom in its ground state is called ionization enthalpy (ΔiH).
X(g) ——> X+(g) + e− ΔiH
Remember : Ionization enthalpy is always positive as energy has to be supplied to remove outer electron from an atom.
Factors on which ionization enthalpy depends are :
- Radius of an atom
- Nuclear charge
- The screening effect of inner electrons
- Nature of electronic configuration.
Pattern of Ionization enthalpy variation along period and along group :
(A) In the periods :
- The elements' first ionisation enthalpy increases from left to right.
- Across the period as the atomic number increases, the electrons are added successively to the same principal shell.
- The nuclear charge increases from left to right in the periods.
- Hence the added electrons poorly shield each other from the nucleus.
- As a result effective nuclear charge increases and causes the decrease in the atomic radii.
Hence, along a period ionization enthalpy increases. In each period, the last inert element has the highest ionization enthalpy, and alkali metals have the lowest value of first ionization enthalpy.
(B) In a group :
- Along a group from top to bottom, first ionization enthalpy decreases.
- As the atomic number increases down the group, the valence electron enters a new principal shell, increasing the atomic radius.
- As the number of inner electrons increases the screening or shielding effect also increases.
- Hence, the force of nuclear attraction on valence electrons decreases and it becomes easier to remove the outermost electrons.
- Therefore, along the group from top to bottom ionization enthalpy decreases, with the increase in atomic number.
Explain the factors affecting ionization enthalpy :
Ans. The magnitude of ionization enthalpy depends upon the following factors :
(1) Size (radius) of an atoms : The nuclear attraction on the outermost electrons decreases as the size of an atom increases. As a result, removing electrons from it is easier. Therefore, as atomic size increases, ionization enthalpy decreases.
(2) Nuclear charge : The greater the charge on an atom's nucleus, the greater the nuclear attraction on the outermost electrons, making it more difficult to remove an electron from an atom. Hence, as the nuclear charge increases, ionization enthalpy increases.
(3) The shielding or screening effect of inner electrons :
- The nuclear attraction on the outermost electrons decreases with the increase in shielding or screening effect.
- Due to screening effect of inner electrons, the effective nuclear charge and an attraction decrease.
- The shielding effect of inner electrons for the given quantum number decreases in the order of s > p > d > f, hence the ionization enthalpy also decreases in the same order.
(4) Nature of electronic configuration : Elements with extra stability due to partially or completely filled orbitals have higher ionisation enthalpy values. As a result, inert elements with full octet and extra stability have high ionisation enthalpies.
First ionization enthalpy : The energy required to remove first electron from a gaseous atom of an element is called first ionization enthalpy.
X(g) ——> X+(g) + e− Δi1H
Second ionization enthalpy : The energy required to remove an electron from singly positively charged gaseous cation (i.e., second electron) of an element is called second ionization enthalpy.
X+(g) ——> X2+(g) + e− Δi2H
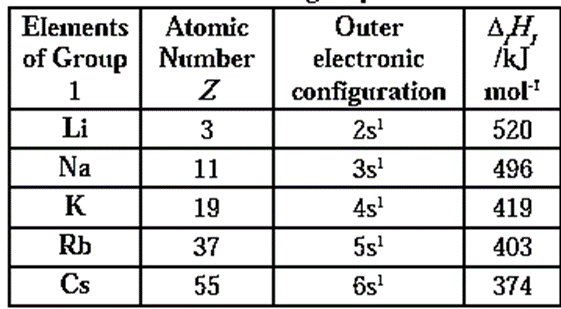
First ionization enthalpy values of elements of group 1 :
First ionization enthalpy values of elements of period 2
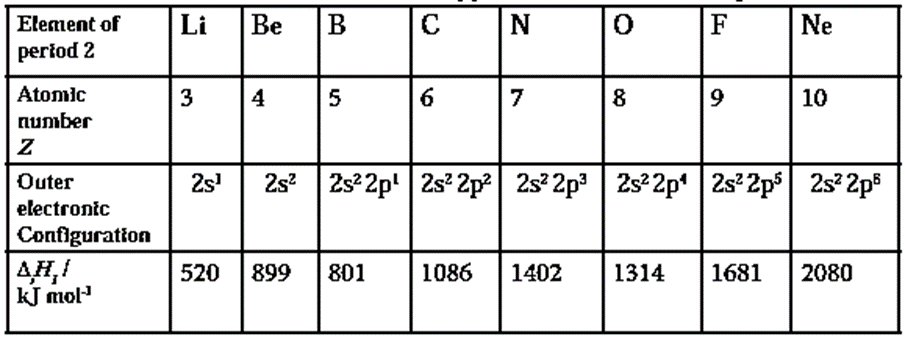
- The electronic configuration of boron (B, Z = 5) is 1s2, 2s2, 2p1 and that of beryllium (Be, Z = 4) is 1s2, 2s2 Due to paired electrons in 2s-orbital, Be acquires extra stability but boron has only one unpaired electron in p—orbital. Thus less energy is required to remove a p-electron than a paired s—electron in the same principal shell. Therefore, ionization enthalpy of boron is less than beryllium.
- First electron is removed from a gaseous neutral atom while second electron is removed from positively charged gaseous cation. Hence the second ionization enthalpy (IE2) is higher than the first ionization enthalpy (IE1).
- Alkali metals have large volume and only one electron in the outermost shell (ns1). Therefore, less energy is required to remove the single unpaired electron from the valence shell. Hence alkali metals have low ionization energy.
Inert gases have exceptionally high ionization potential :
Reason :
- Noble gases have completely filled outermost shell.
- They have completely filled orbitals with complete octet (ns2 np6) and thus they acquire extra stability (except He with two electrons).
- After a loss of electron, noble gas forms unstable cation.
- Hence more energy is required to remove the outermost electron.
- Therefore, noble gases have high values of ionization enthalpy.
Electron gain enthalpy:
The enthalpy change that takes place when an electron is added to an isolated gaseous atom in its ground state is called the electron gain enthalpy, ΔegH.
X(g) + e− ——> X−(g) ΔegH
(This is also known as electron affinity.)
Trends or variation in electron gain enthalpy in the periodic table :
The variation in electron gain enthalpy of the elements is less systematic. The process of adding electron to a gaseous atom may be endothermic or exothermic depending upon the nature of the element.
(A) In a period :
- The electron gain enthalpy becomes more negative with the increase in atomic number across a period.
- This is due to the increase in nuclear charge across the period from left to right.
- Atomic size decreases from left to right hence the tendency to capture an added electron increases.
- Therefore, electron gain enthalpy (electron affinity) increases across the period.
(B) In a group :
- Electron gain enthalpy becomes less negative from top to bottom in the group.
- The atomic size of the elements in a group increases from top to bottom, since the last electron enters a new outermost shell.
- The nuclear attraction on the added electron decreases due to a large screening effect.
- Therefore, electron gain enthalpy (electron affinity) decreases down the group.
Table of Electron gain enthalpies of some main group elements:
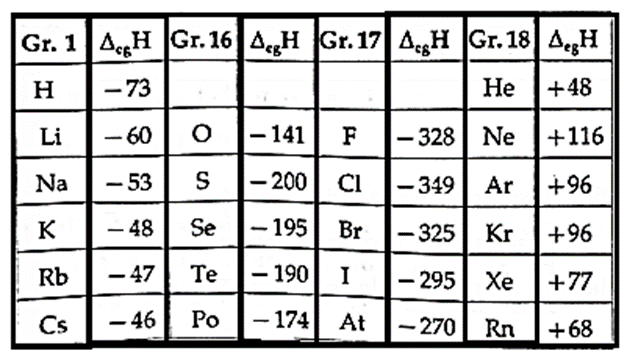
- Halogens (F, Cl, Br, I, At) in group 17 have very high negative values of electron gain enthalpy as they acquire stable electronic configuration of noble gas on addition of one electron.
- Noble gases in group 18 show high positive values of electron gain enthalpy because the added electron has to enter the next higher shell. This is very unstable electronic configuration. (due to high shielding effect and low effective nuclear charge.)
- The alkali metals have very low negative electron gain enthalpy values.
Remember : Negative value of electron gain enthalpy indicates more tendency to gain an electron while positive value indicates less tendency to gain or accept an electron.
Electronegativity (EN) : Electronegativity is the ability of an atom in a compound to attract shared electrons of a covalent bond to itself.
| Electronegativity | Electron gain enthalpy |
| Electronegativity of any given element is not constant, but depends upon the other element to which is bound in a compound. | Electron gain enthalpy of an element is constant. |
| Electronegativity cannot be measured experimentally. It is obtained from numerical scale of electronegativity developed mainly by Linus Pauling. | Electron gain enthalpy is a measurable property.
|
Trends or variation in electronegativity :
(A) In a period :
- In a period electronegativity increases from left to right. (e.g. Li < Be < B < C < N < O < F).
- In a period, as atomic number increases atomic radius (size) decreases, and nuclear charge increases from left to right.
- Hence due to the increase in nuclear attraction, the tendency to attract shared electron pair in a covalent bond increases from left to right. Therefore, electronegativity increases.
(B) In a group :
- In a group, electronegativity decreases from top to bottom (e.g. F > Cl > Br > I > At).
- In a group, as atomic number increases, outermost principal shell number increases, hence atomic radius (size) increases, and thus nuclear attraction decreases.
- The tendency to attract shared electron pair of a chemical bond decreases, decreasing the electronegativity down the group.
Electronegativity (EN) values of some elements (Pauling scale) :

Periodic trends in chemical properties:
Valency : Valency of the elements is usually equal to number of valency electrons (outer electrons) or equal to difference between 8 and number of valence electrons.
Oxidation state : oxidation number or oxidation state of an element in a particular compound is the apparent charge acquired by its atom on the basis of electronegativity of other atoms in a molecule of a compound. It can be positive or negative (+,− ) or zero.
Periodic trends in valency of main group elements :

Periodic trends in valency:
- In periods :In period as atomic number increases from left to right, the valence increases from 1 to 4 and then decrease from 4 to zero.
- In groups : Down the group valence remains same as all the elements possess the same number of valence electrons.
Periodic trends in metallic–nonmetallic character :
- In period :As ionization enthalpy increases across a period from left to right, the metallic character decreases. Since electron gain enthalpy becomes more negative across a period, nonmetallic character increases.
- In group :As ionization enthalpy decreases down the group, tendency to lose valence electrons increase. Hence, metallic character increases and nonmetallic character decreases.
Periodic trends in chemical reactivity:
- The ease with which an element loses or gains electrons is related to its chemical reactivity.
- Elements on the far left of the periodic table have the lowest ionisation potential (for example, group 1, alkali metals) and are highly reactive, electropositive metals.
- Elements on the far right have the most negative electron gain enthalpy (for example, halogens in group 17) and are highly reactive nonmetals.
- Transition and inner transition elements are all metals with similar chemical properties due to small changes in atomic radii. They have ionisation enthalpy values that are intermediate between s—block and p-block elements.
Chemical properties of metals and nonmetals :
- Metals react with oxygen to form corresponding oxides which are basic in nature. The oxides of reactive metals when dissolved in water give base. For example, sodium form sodium oxide (Na2O) which form a base (NaOH) with water.
- The nonmetals react with O2 to form acidic oxides. The oxides of nonmetals form acids when dissolved in water. For example, oxide of reactive nonmetals like chlorine form Cl2O7 which forms strong acid (HClO4) with water.
- The oxides of the elements in the center of the main group elements form amphoteric oxides of metals (Al2O3) or neutral or weakly acidic oxides of nonmetal. For example, CO and NO are neutral whereas CO2 is weakly acidic.
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6-Redox Reactions – Online Notes
Next Chapter : Chapter-8-Elements of Groups 1 and 2 – Online Notes
We reply to valid query.