Biomolecules
Maharashtra Board-Class-12-Chemistry-Chapter-14
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction : Principal molecules of the living world :
Biomolecules : Biomolecules are the lifeless molecules which combine in specific manner to produce life or control biological reactions.
- Examples : They are carbohydrates, lipids, proteins, nucleic acids. They play an important role in functions of organisms.
Importance of biomolecules :
- Biomolecules are organic molecules that combine in certain ways to form complex compounds that are essential to an organism's ability to function, create daughter cells that are exactly like the parent cell, and support life.
- The main component of food and a source of energy is carbohydrates.
- Proteins are essential to the healthy operation of living things. They are crucial components of muscles, skin, and hair. Proteins are the building blocks of enzymes, which catalyze chemical reactions within cells.
- Fats and oils, or lipids, serve as the body's energy reserves.
- In living things, enzymes function as biological catalysts for a variety of chemical reactions. Tiny amounts of these are needed.
Carbohydrates :
Carbohydrates : Carbohydrates are optically active polyhydroxy aldehydes or polyhydroxy ketones, or the compounds which on hydrolysis produce polyhydroxy aldehydes or polyhydroxy ketones.
- Examples : Glucose. sucrose. fructose.
Classification of carbohydrates :
Carbohydrates are classified as monosaccharides oligosaccharides and polysaccharides.
(I) Monosaccharides : These carbohydrates cannot be further hydrolysed into smaller units. They are basic units of all carbohydrates, and are called monosaccharides.
- Examples : Glucose, fructose, ribose
(II) Oligosaccharides : An oligosaccharide is a carbohydrate (sugar) which on hydrolysis gives two to ten monosaccharide units.
Depending on the number of monosaccharides produced on hydrolysis, oligosaccharides are further classified as :
(i) Disaccharides (C12H22O11) :
Sucrose \(\underrightarrow{Hydrolysis}\) Glucose + Fructose
Maltose \(\underrightarrow{Hydrolysis}\) Glucose + Glucose
Lactose \(\underrightarrow{Hydrolysis}\) Glucose + Galactose
(ii) Trisaccharides (C18H32O16) :
Raffinose \(\underrightarrow{Hydrolysis}\) Glucose + Fructose + Galactose
(iii) Tetrasaccharides (C24H42O21) :
Stachyose \(\underrightarrow{Hydrolysis}\) Glucose + Fructose + Two Galactose
Oligosaccharide is homogeneous. In this, each molecule of oligosaccharide contains the same number of monosaccharide units joined together in the same order as every other molecule of the same oligosaccharide.
Polysaccharides : These are carbohydrates which on hydrolysis give a large number of monosaccharides.
- Polysaccharides are tasteless, amorphous, insoluble in water. They are long chain, naturally occurring polymers of
- Examples : Cellulose, starch, glycogen.
Remember :
- About twenty different monosaccharides are found in carbohydrates.
- Disaccharides are the most common oilgosacchrides. The two monosaccharide units in disaccharides may be same or different.
- Polysaccharides : Starch is common ingredient of food grains. Cellulose is constituent of cell wall of plant cells. Animals store in their body in the form of glycogen.
Classification of carbohydrates :

Nomenclature of monosaccharides :
- According to IUPAC system of nomenclature, general name for monosaccharide is glucose. Monosaccharide with one aldehydic carbonyl group is called aldose while that with one ketonic carbonyl group is called ketose.
- These names are further modified in accordance with the total number of carbon atoms in the monosaccharide.
- For example, glucose (C6H12O6) is an aldose with six carbons, and is thereby, an aldohexose.
- Fructose (C6H12O6) is a ketose with six carbons, and is, thereby, a ketohexose.
Examples :
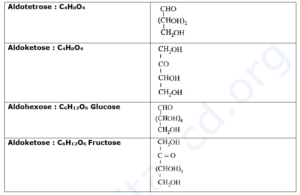
Glucose :
Glucose occurs in nature in free as well as in combined state. Glucose can be obtained from sucrose or starch by acid catalysed hydrolysis.
(i) Prepartion of glucose from sucrose : Laboratory method.
- Glucose is prepared in the laboratory by hydrolysis of sucrose by boiling it with dilute hydrochloric acid or dilute sulphuric acid for about two hours.
- On hydrolysis, sucrose gives one molecule of glucose and one molecule of fructose.
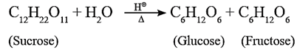
- Alcohol is added during cooling to separate glucose and fructose since, glucose is almost insoluble in alcohol, hence it crystallizes out first.
- Fructose remains in the solution as it is more soluble than glucose.
- Crystals of glucose are separated out by filtration and purified by recrystallization.
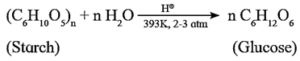
(ii) Prepartion of glucose from starch :
Commercially glucose is obtained by hydrolysis of starch by boiling it with dilute sulfuric acid at 393K under 2 to 3 atm pressure. Starch is hydrolysed to give glucose.
Structure and properties of glucose :
- Glucose has an aldohexose structure. In other words, glucose molecule contains one aldehydic, that is, formyl group and the remaining five carbons carry one hydroxyl group (-OH) each. The six carbons in glucose form one straight chain.
- This aldohexose structure of glucose was established on the basis of the following chemical properties.
(i) Molecular formula of glucose was found to be C6H12O6, on the basis of its elemental composition and colligative properties.
(ii) Action of HI : Glucose on prolonged heating with HI gives n-hexane, indicates that all the six carbon atoms are linked in straight chain.
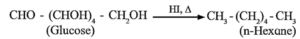
(ii) Action of hydroxyl amine : Glucose reacts with hydroxyl amine in an aqueous solution to form glucose oxime. This indicates the presence of CHO group in glucose.

(iii) Action of hydrogen cyanide : Glucose reacts with hydrogen cyanide to form glucose cyanohydrin.
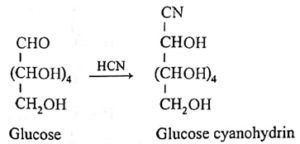
(iv) Action of bromine water : Glucose on oxidation with mild oxidising agent like bromine water gives gluconic acid, which shows that the carbonyl group in glucose is aldehyde group.

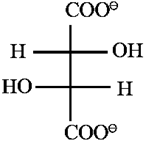
(v) Glucose contains one primary alcoholic (- CH2OH) group : Action of dil. nitric acid : Glucose on oxidation with dilute nitric acid forms dicarboxylic acid, saccharic acid, This indicates the presence of a primary alcoholic group (—CH2OH) in glucose.

(vi) Glucose contains five hydoxyl groups : Action of acetic anhydride : When glucose is heated with acetic anhydride in the presence of catalyst pyridine, glucose penta acetate is formed. It indicates that glucose is a stable compound and contains five hydroxyl groups.

Optical isomerism in glucose :
Structural formula of glucose contains four chiral carbon atoms. Every chiral carbon can have two distinct spatial arrangements of groups around it. In other words, two distinct configurations are possible for each of the four chiral carbons of glucose. Stereostructure of glucose is therefore one out of several possible stereostructures of an aldohexose.
Emil Fischer, a German Nobel laureate (1902), determined the configuration of the four chiral carbons (C-2, C-3, C-4, C-5) in glucose.
Fischer projection formulae of glucose, gluconic acid and saccharic acid :

| Know This :
(1) A structural formula containing ‘n’ number of chiral carbon can have maximum ‘2n’ numbers of stereostructures or optical isomers. An aldohexose therefore, can exist as sixteen (24 = 16) optical isomers, and glucose is one of them. (2) Optical rotation is an experimentally measurable property of a compound. Configuration of chiral carbon, on the other hand, is difficult to observe by simple experiment. In 1951 X-ray crystallographic studies of (+) -sodium rubidium tartarate established its configuration as :
This was the first instance of determining absolute configuration. |
D and L configuration in sugars :
The simplest carbohydrates glyceraldehyde is chosen as the standard, to assign D and L configuration to monosaccharides. Glyceraldehyde contains one asymmetric carbon atom and exist in two enantiomeric forms.
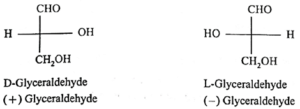
- The dextro enantiomer is represented as (+) glyceraldehyde and it is referred as D-configuration i.e., D-glyceraldehyde. The laevo enantiomer of glyceraldehyde is represented as (—) glyceraldehyde and it correlated as L-configuration i.e., L-glyceraldehyde.
- In Fischer projection formula, a monosaccharide is assigned D-configuration if the (—OH) hydroxyl group at the last chiral carbon and lies towards right hand side. On the other hand it is assigned L-configuration if the —OH group on the last chiral carbon atom and lies on the left hand side. In monosaccharides, the most oxidized carbon (i.e., —CHO) is at the top.
Examples :
(i)

(ii) Fischer projection formulae for (a) L—(+)-erythrose (b) L—(+) ribulose.
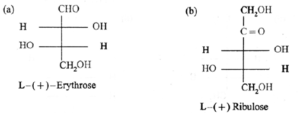
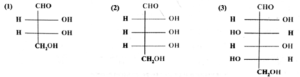
Q. Assign D/L configuration to the following monosaccharides.

Ring structure of glucose :
Glucose is found to have two cyclic structures (VI and VII) which are in equilibrium with each other through the open chain structure (I) in aqueous solution (see below Fig.).

- The ring structure of glucose is formed by reaction between the formyl (-CHO) group and the alcoholic (-OH) group at C-5. Thus, the ring structure is a hemiacetal
- The two hemiacetal structures (VI and VII) differ only in the configuration of C-1 (Fig.), the additional chiral centre resulting from ring closure.
- The two ring structures are called ∝- and β- anomers of glucose and C-1 is called the anomeric carbon.
- The ring of the cyclic structure of glucose contains five carbons and one oxygen. Thus, it is a six membered ring. It is called pyranose structure, in analogy with the six membered heterocyclic compound pyran (VIII). Hence glucose is also called glucopyranose.
Examples :
(i) Structures of α —D-( + )-glucopyranose and β —D—( + )—glucopyranose.
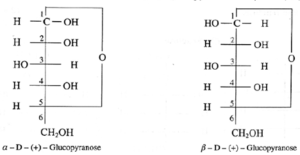
Haworth formula of glycopyranose : Haworth formula is a better way than Fischer projection formula to represent structure of glucopyranose (see below Fig.).
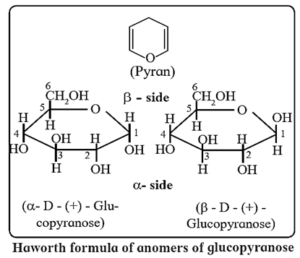
- In the Haworth formula the pyranose ring is considered to be in a perpendicular plane with respect to the plane of paper.
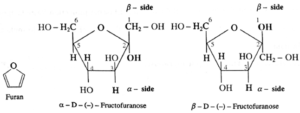
- The carbons and oxygen in the ring are in the places as they appear in above Fig. The lower side of the ring is called α-side and the upper side is the β-side. The α-anomer has its anomeric hydroxyl (-OH) group (at C-1) on the ∝-side, whereas the β-anomer has its anomeric hydroxyl (-OH) group (at C-1) on the β-side.
- The groups which appear on right side in the Fischer projection formula appear on a-side in the Haworth formula, and viceversa.
Reducing nature of glucose : Hemiacetal group of glucopyranose structure is a potential aldehyde group. It imparts reducing properties to glucose. Thus, glucose gives positive Tollens test and positive Fehling test.
Representation of Fructose structure :
Fructose has molecular formula C6H12O6. It contains ketonic functional group at carbon number 2 and six carbon atoms in straight chain. It belongs to D—series and is a laevo rotatory compound. It is written as D—(—)—fructose.
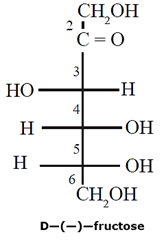
Being an α-hydroxy keto compound fructose is a reducing sugar. In the free state fructose exists as mixture of fructopyranose (major) and fructofuranose. In combined state it is found in the form of fructofuranose ring structure.
Examples :
(i) Mirror images of glucose and fructose :
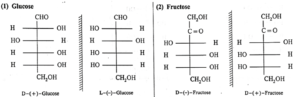
(ii) Two cyclic structures of α—D—(—)—fructofuranose and β—D—(—)—fructofuranose exist in equilibrium with open chain structure :
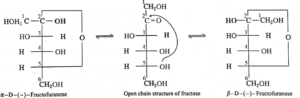
(iii) Haworth projection formulae for α—D—(—)—Fructofuranose and β—D—(—)—Fructofuranose.
Disaccharides :
- Disaccharides give rise to two units of same or different monosaccharides on hydrolysis with dilute acids or specific enzymes.
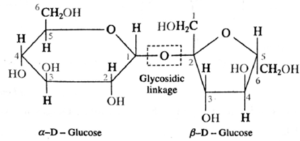
- The two monosaccharide units are linked together by an ether oxide linkage (-O-), which is termed as glycosidic linkage in carbohydrate chemistry.
- Glycosidic linkage is formed by removal of a water molecule by reaction of two hydroxyl (-OH) groups from two monosaccharide units.
- At least one of the two monosaccharide units must use its anomeric hydroxyl group in formation of the glycosidic linkage.
- Three most common disaccharides are sucrose, maltose and lactose.
(i) Structure of sucrose :
Sucrose is a disaccharide and has molecular formula C12H22O11. The structure of sucrose contains glycosidic linkage between C—1 of α-glucose and C-2 of β-fructose. Since aldehyde and ketone groups of both monosaccharide units are involved in the formation of glycosidic bond, sucrose is a nonreducing sugar.
Sucrose is dextro rotatory, on hydrolysis with dilute acid or an enzyme invertase gives equimolar mixture of dextro rotatory glucose and laevo rotatory fructose.
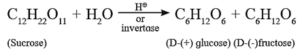
The solution is laevo rotatory because laevo rotation of fructose (-92.4°) is more than dextro rotation of glucose ( + 52.50), hence the sign of rotation is changed from (+) to (-) after hydrolysis, the product is called invert sugar.
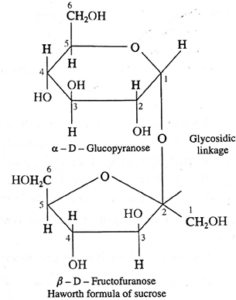
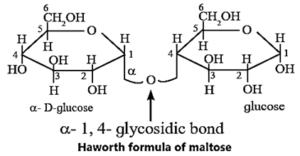
(ii) Structure of maltose :
Maltose is another disaccharide obtained by partial hydrolysis of starch or made of two units of D—glucose. In maltose, C—1 of one α—D-glucose is linked to C-4 of another α—D-glucose molecule by glycosidic linkage.
The glucose ring which uses its hydroxyl group at C-1 is α-1→ 4 glycosidic linkage. It is a reducing sugar because a free aldehyde group bond can be produced at C, of second glucose molecule. Maltose on hydrolysis with dilute acids gives glucose.
| Know This :
Invert sugar is commercially available as invert syrup. It is used as sweetene in bakery and confectionary products and also in fruit preserves and beverages. It is sweeter than sucrose and glucose. It is resistant to crystallization and promotes retention of moisture, enhances flavour and texture and also prolongs shelf life. |
(iii) Structure of lactose :
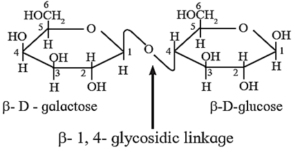
- Lactose (C12H22O11) is a disaccharide. It is found in milk, therefore, it is also known as milk sugar.
- It is formed from two monosaccharide units, namely D-galactose and D—glucose. The glycosidic linkage is formed between C—1 of β—D-galactosc and C—4 of glucose. Therefore, the linkage in lactose is called β-1,4—glycosidic linkage.
- The hemiacetal group at C—1 of the glucose unit is not involved in glycosidic linkage but is free. Hence lactose is a reducing sugar. The above figure shows Haworth formula of lactose.
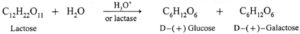
Hydrolysis products of lactose : Lactose on hydrolysis in presence of an acid or enzyme lactase gives one molecule each of glucose and galactose
Polysaccharides :
- Polysaccharides are formed by linking large number of monosaccharide units by glycosidic linkages.
- Starch, cellulose and glycogen are the most common natural polysaccharides.
(i) Structure of starch :
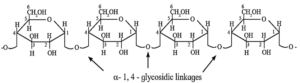
Starch is found in cereal grains, roots, tubers, potatoes, etc. It is a polymer of α—D—glucose and consists of two components, amylose and amylopectin.
(i) Amylose is water soluble component forms blue coloured complex with iodine. It constitutes about 20 % of starch. Amylose contains 200 to 1000 α—D—glucose units linked together by glycosidic linkage between C—1 of one unit and C—4 of another unit. i.e. α—1, 4 glycosidic linkages.
(ii) Amylopectin is insoluble in water and constitutes about 80 % starch which forms blue-violet coloured complex with iodine. It is a branched chain polymer. In amylopectin, α—D—glucose molecules are linked together by glycosidic linkage between C1—of one unit and C—4 of another unit to form long chain and branching occurs by glycosidic linkage between C—1 and C6 glycosidic linkage.
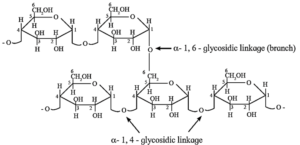
Polysaccharides :
A large number of same or different monosaccharides are joined together by glycosidic linkages are called polysaccharides. They have general formula (C6H10O5)n.
- Examples : Starch, cellulose, glycogen, etc.
Structure of cellulose :
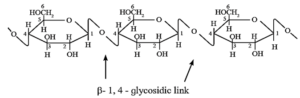
Cellulose mainly occurs in plants. Cell wall of plant cells is made up of cellulose.
It is a long chain polymer. In cellulose, β-D-glucose units are linked by glycosidic linkage between C1 of one unit of glucose and C4 of another glucose unit.
Thus cellulose contains 1 — 4 β glycosidic linkages like those in cellobiose. Cellulose
is hydrolysed using strong acids at high temperature and pressure. This implies that
the β—1, 4—glycosidic bond is very strong and difficult to hydrolyses.
Structure of glycogen :
The glucose is stored in animal body in the form of glycogen. It is also known as animal starch because its structure is similar to amylopectin. Glycogen is highly branched. Whenever the body is required glucose, enzymes breaks the glycogen to glucose.
Know This :
|
Remember :
- Starch is the main storage molecules of plants whereas glycogen is the main storage molecule of animals. Starch is found in cereals, roots, tubers, etc. Glycogen is present in liver, muscles and brain.
Basic structural difference between starch and cellulose :
- Starch is a polymer of a-glucose and consists of two components—amylose and amylopectin.
- In amylose α—D—D—( +)—glucose units held by C1—C4 glycosidic linkage and in amylopectin, α—D—glucose units held by C1—C4 glycosidic linkage whereas branching occurs by C1—C6 glycosidic linkage.
- Cellulose is a straight chain polysaccharide composed only of β—D—glucose units held by C1—C4 glycosidic linkage.
PDF : Class-12-Chemistry-Chapter-14-Biomolecules-Text Book
PDF : Class-12-Chemistry-Chapter-14-Biomolecules- Notes
PDF : Class-12-Chemistry-Chapter-14-Biomolecules- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF) Rs.137-Buy
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 13- Amines – Online Notes
Next Chapter : Chapter-15-Introduction to Polymer Chemistry– Online Notes
We reply to valid query.