Some Basic Concepts of Chemistry
Class-11-Science-Chemistry-Chapter-1-Maharashtra State Board
Solution
Question 1.
Choose the most correct option.
(A) A sample of pure water, whatever the source always contains by mass of oxygen and 11.1 % by mass of hydrogen.
(a) 88.9 (b) 18 (c) 80 (d) 16
(a) 88.9
(B) Which of the following compounds can NOT demonstrate the law of multiple proportions ?
(a) NO, NO2
(b) CO, CO2
(c) H2O, H2O2
(d) Na2S, NaF
(d) Na2S, NaF
(C) Which of the following temperature will read the same value on celsius and
Fahrenheit scales.
(a) - 400
(b) + 400
(c) -800
(d) -200
(a) - 400
(D) SI unit of the quantity electric current is
(a) Volt (b) Ampere (c) Candela (d) Newton
(b) Ampere
(E) In the reaction N2 + 3H2 → 2NH3, the ratio by volume of N2, H2 and NH3 is 1 : 3 : 2 This illustrates the law of
(a) definite proportion
(b) reciprocal proportion
(c) multiple proportion
(d) gaseous volumes
(d) gaseous volumes
(F) Which of the following has maximum number of molecules ?
(a) 7 g N2
(b) 2 g H2
(c) 8 g O2
(d) 20 g NO2
(b) 2 g H2
(G) How many g of H2O are present in 0.25 mol of it?
(a) 4.5
(b) 18
(c) 0.25
(d) 5.4
(a) 4.5
(H) The number of molecules in 22.4 cm3 of nitrogen gas at STP is
(a) 6.022 x 1020
(b) 6.022 x 1023
(c) 22.4 x 1020
(d) 22.4 x 1023
(a) 6.022 x 1020
(I) Which of the following has the largest number of atoms ?
(a) 1g Au (s)
(b) 1g Na (s)
(c) 1g Li (s)
(d) 1g Cl2 (g)
(c) 1g Li (s)
Question 2.
Answer the following questions.
(A) State and explain Avogadro's law.
Avogadro’s law : It states that equal volumes of different gases under identical conditions of pressure and temperature contain equal number of molecules. Explanation: Consider the reaction between gaseous hydrogen and oxygen forming water vapour. 2H2(g) + O2(g) à 2H2O(g) 100 mL 50 mL 100 mL 2 volumes 1 volume 2 volumes Since volume V of a gas is directly proportional to number of gas molecule (Vµn), 2 n molecules : n molecule : 2 n molecules OR 2 molecules : 1 molecule : 2 molecules Thus 2 molecules of hydrogen combine with 1 molecule of oxygen to give 2 molecules of water vapour.
(B) Point out the difference between 12 g of carbon and 12 u of carbon.
12 g of carbon represents one mole of carbon containing 6.022 x 1023 atoms of carbon. 12 u of carbon represents one carbon atom.
(C) How many grams does an atom of hydrogen weigh ?
Atomic mass of hydrogen is 1.0 u. Hence its mass is 1.0 x 1.66054 x 10-24 g = 1.66054 x 10-24 g.
(D) Calculate the molecular mass of the following in u.
(a) NH3 (b) CH3COOH (c) C2H5OH
(a) NH3 = 14u + 3 x 1u = 17u. (b) CH3COOH = 2 x 12u + 4 x 1u + 2 x 16u = 60u. (c) C2H5OH = 2 x 12u + 6 x 1u + 16u = 46u
(E) How many particles are present in 1 mole of a substance ?
One mole of a substance contains 6.022 x 1023 particles.
(F) What is the SI unit of amount of a substance ?
The SI unit of amount of a substance is ’mol'
(G) What is meant by molar volume of a gas ?
Molar volume of a gas : The volume of one mole of a gas is called molar volume. At STP condition (0°C and 1 atm), the molar volume of any gas is 22.4 dm3.
(H) State and explain the law of conservation of mass.
Law of conservation of mass : This law states that during chemical combination of matter, the mass is neither created nor destroyed. Explanation : Antoine Lavoisier a French scientist while studying several combustion reactions, determined accurately the masses of the reactants before reactions and the masses of the products after the completion of the reactions. He observed that the total masses of the reactants before the reaction is equal to the total masses of the products. For example 1) When hydrogen gas burns and combines with oxygen to yield water, the mass of the water formed is equal to the mass of the hydrogen and oxygen consumed. Hydrogen + Oxygen —> Water 2 g 16 g 18 g 2) When solid carbon burns and combines with oxygen to form carbon dioxide, The total mass of carbon (12 g) and oxygen taken initially (32 g) is equal to the mass of Carbon dioxide (44 g) formed. C + O2 —> CO2 (s) (g) (g) (12g) (32g) —> (44g) Applications :
(I) State the law of multiple proportions.
The law of multiple proportions : This law states that when two elements chemically combine to form two or more compounds with different compositions by weights, then the masses of one element that combine with a fixed mass of the other element are in the ratio of small whole numbers. Explanation : Hydrogen combines with oxygen to form two compounds, namely water (H2O) and hydrogen peroxide (H2O2). Hydrogen + Oxygen ——> Water 2 g 16 g 18 g Hydrogen + Oxygen -—> Hydrogen Peroxide 2 g 32 g 34 g It is found that the two masses of oxygen namely 16 g and 32 g which combine with a fixed mass of hydrogen (2 g) are in the ratio of small hole numbers, i.e. 16 : 32 or 1 : 2
Question 3.
Give one example of each
(A) homogeneous mixture
An aqueous solution of sugar
(B) heterogeneous mixture.
A mixture of oil and water.
(C) element.
Copper -Cu is an element.
(D) compound
Water, H2O is a compound.
Question 4.
Solve problems :
(A) What is the ratio of molecules in 1 mole of NH3 and 1 mole of HNO3.
1 mole of NH3 and 1 mole of HNO3 contain 6.022 x 1023 molecules each. Hence the ratio of molecules is 1 : 1.
(B) Calculate number of moles of hydrogen in 0.448 liter of hydrogen gas at STP.
At STP, 22.4 litre of H2 contains 1 mol of H2 ∴ At STP 0.448 litre of H2 will contain, 0.448/22.4 = 0.02 mol of H2 Answer is - Moles of H2 = 0.02 mol.
(C) The mass of an atom of hydrogen is 1.008 u. What is the mass of 18 atoms of hydrogen?
Mass of hydrogen atoms = 18 x 1.008 = 18.144 u Answer is -Mass of hydrogen atoms = 18.144 u.
(D) Calculate the number of atom in each of the following (Given : Atomic mass of I = 127 u).
(a) 254 u of iodine (I)
(b) 254 g of iodine (I)
(a) Number of atoms of iodine (I) = 254/127 = 2 atoms (b) Mass of 1 mol of I atoms = 127 x 6.022 x 1023 u = 127 x 6.022 x 1023 X 1.66054 x 10−24 g = 127 g mol−1 ∴ Number of moles of I = 254/127 = 2 mol ∴ Number of I atoms = 2 x 6.022 x 1023 = 1.2044 x 1024 atoms Answer is- (a) Number of I atoms = 2 (b) Number of I atoms = 1.2044 x 1024
(E) A student used a carbon pencil to write his homework. The mass of this was found to be 5 mg. With the help of this calculate.
(a) The number of moles of carbon in his homework writing.
(b) The number of carbon atoms in 12 mg of his homework writing.
(a) Number of moles of carbon = \(\frac{5×10^{-3}}{12}\) = 4.166 x 10−4 mol (b) Number of moles of carbon = \(\frac{12×10^{-3}}{12}\) = 1 x 10−3 mol ∴ Number of carbon atoms = 1 x 10−3 x 6.022 x 1023 = 6.022 x 1020 Answer is- (a) Number of moles of carbon = 4.166 x 10−4 mol (b) Number of carbon atoms = 6.022 x 1020
(F) Arjun purchased 250 g of glucose (C6H12O6) for Rs 40. Find the cost of glucose per mole.
Molar mass of glucose (C6H12O6) = 6 x 12 + 12 x 1+ 6 x 16 = 180 g mol−1 250 g of glucose costs Rs. 40 ∴ 180 g of glucose will cost, = \(\frac{40×180}{250}\) = 28.8 Answer is - Cost of glucose per mole = Rs. 28.8.
(G) The natural isotopic abundance of 10B is 19.60% and 11B is 80.40 %. The exact isotopic masses are 10.013 and 11.009 respectively. Calculate the average atomic mass of boron.
Consider a boron sample containing 19.60% of 10B and 80.40% of 11B isotopes. Average atomic mass of boron = \(\frac{19.60×10.013+80.40×11.0009}{100}\) = \(\frac{196.2548+885.1236}{100}\) = 10.813784 ≅ 10.81 u Answer is- Average atomic mass of boron = 10.81 u
(H) Convert the following degree Celsius temperature to degree Fahrenheit.
(a) 40 0C (b) 30 0C
(a) \(\frac{°C}{5}=\frac{°F-32}{9}\) ∴ 0F = \(\frac{9×°C}{5}+32\)=(\frac{9×40}{5}+32\) = 72 + 32 =104 0F (b) 0F = \(\frac{9×°C}{5}+32\)=(\frac{9×30}{5}+32\) = 54 + 32 = 86 0F Answer is – (a) 104 0F (b) 86 0F
(I) Calculate the number of moles and molecules of acetic acid present in 22 g of it.
Molar mass of acetic acid (CH3COOH) = 60 g mol−1 Number of moles of CH3COOH = n = W/M = 22/60 = 0.3660 mol Number of molecules of CH3COOH = n x NA = = 0.3660 x 6.022 x 1023 = 2.2076 x 1023 Answer is- Number of moles of CH3COOH = 0.3660 mol Number of molecules of CH3COOH = 2.2076 x 1023
(J) 24 g of carbon reacts with some oxygen to make 88 grams of carbon dioxide. Find out how much oxygen must have been used.
Mass of carbon + Mass of oxygen = Mass of CO2 Mass of oxygen = Mass of CO2 — Mass of carbon = 88 − 24 = 64 g Answer is- Mass of oxygen used = 64 g.
(K) Calculate number of atoms is each of the following. (Average atomic mass : N = 14 u, S = 32 u)
(a) 0.4 mole of nitrogen
(b) 1.6 g of sulfur
(a) Number of molecules of N2 = 0.4 x 6.022 x 1023 ∴ Number of N atoms = 0.4 x 6.022 x 1023 x 2 = 4.8176 x 1023 (b) Number of moles of S = W/M = \(\frac{1.6}{32}\) mol ∴ Number of atoms of S = \(\frac{1.6}{32}\) x 6.022 x 1023 = 3.011 x 1022 Answer is- (a) Number of N atoms = 4.8176 x 1023 (b) Number of atoms of S = 3.011 x 1022
(L) 2.0 g of a metal burnt in oxygen gave 3.2 g of its oxide. 1.42 g of the same metal heated in steam gave 2.27 of its oxide. Which law is verified by these data ?
Mass of oxygen in metal oxide = 3.2 − 2 = 1.2 g Mass of oxygen in second case = 2.27 − 1.42 = 0.85 g 0.85 g oxygen combines with 1.42 g metal ∴ 1.2 g oxygen will combine with, \(\frac{1.42×1.2}{0.85}\) = 2.0 g metal. This observation represents a law of definite proportion.
(M) In two moles of acetaldehyde (CH3CHO) calculate the following
(a) Number of moles of carbon
(b) Number of moles of hydrogen
(c) Number of moles of oxygen
(d) Number of molecules of acetaldehyde
(a) 1 mol of acetaldehyde, CH3CHO contains 2 mol of carbon ∴ 2 mol of acetaldehyde contains 2 x 2 = 4 mol carbon. (b) 1 mol CH3CHO contains 4 mol H ∴ 2 mol CH3CHO contain 2 x 4 = 8 mol H (c) 1 mol CH3Cl-IO contains 1 mol oxygen ∴ 2 mol CH3CHO contain 2 mol oxygen (d) Number of molecules of CH3CHO = 2 x 6.022 x 1023 = 1.2044 x 1024 molecules Answer is- (a) 4 mol C, (b) 8 mol H (c) 1 mol oxygen (d) 1.2044 x 1024 molecules.
(N) Calculate the number of moles of magnesium oxide, MgO in (i) 80 g and (ii) 10 g of the compound. (Average atomic masses of Mg = 24 and O = 16)
Molar mass of MgO = 24 + 16 = 40 g mol−1 ∴ (i) Number of moles of MgO in 80g = n = W/M = 80/40 = 2 mol (ii) Number of moles of MgO in 10g = 10/40 = 0.25 mol Answer is – (i) Number of moles of MgO = 2 mol (ii) Number of moles of MgO = 0.25 mol
(O) What is volume of carbon dioxide, CO2 occupying by (i) 5 moles and (ii) 0.5 mole of CO2 gas measured at STP.
1 mol CO2 at STP occupies 22.4 dm3 ∴ (i) 5 mol CO2 at STP occupies, 5 x 22.4 = 112 dm3 (ii) 0.5 mol CO2 occupies Volume of CO2 at STP = 0.5 x 22.4 = 11.2 dm3 Answer is- (i) Volume of CO2 = 112 dm3 (ii) Volume of CO2 = 11.2 dm3
(P) Calculate the mass of potassium chlorate required to liberate 6.72 dm3 of oxygen at STP. Molar mass of KClO3 is 122.5 g mol−1.
2KClO3 ——> 2KCl + 3O2 2 mol 3 mol Volume of 3 mol O2 at STP = 3 x 22.4 = 67.2 dm3 ∴ 67.2 dm3 of O2 is liberated at STP by 2 mol KClO3 ∴ 6.72 dm3 of O2 at STP will be liberated by, \(\frac{6.72}{67.2}×2\) = 0.2 mol KClO3 Mass of KClO3 required = 0.2 x 122.5 = 24.5 g Answer is- Mass of KClO3 required =24.5 g.
(Q) Calculate the number of atoms of hydrogen present in 5.6 g of urea, (NH2)2CO. Also calculate the number of atoms of N, C and O.
Molar mass of urea, (NH2)2CO = 60 g mol−1 Number of moles of urea = W/M = 5.6/60 = 0.09333 and ∴ Number of molecules of urea = n x NA = 0.09333 x 6.022 x 1023 = 0.5620 x 1023 ∴ Number of H atoms = 4 x 0.5620 x 1023 = 2.248 x 1023 Number of N atoms = 2 x 0.5620 x 1023 = 1.124 X 11023 Number of C atoms = 1 x 5.620 x 1022 = 5.620 x 1022 Number of O atoms = 1 x 5.620 x 1022 = 5.620 x 1022 Answer is- Number of H atoms = 2.248 x 1023 Number of N atoms = 1.124 x 1023 Number of C atoms = 5.620 x 1022 Number of O atoms = 5.620 x 1022
(R) Calculate the mass of sulfur dioxide produced by burning 16 g of sulfur in excess of oxygen in contact process. (Average atomic mass : S = 32 u, O = 16 u)
S + O2 → SO2 32 g 1 mole 32 g sulphur produces 1 mole SO2 ∴ 16 g sulphur will produce 0.5 mol SO2 Molar mass of SO2 = 64 g mol−1 ∴ Mass of SO2 produced = 0.5 x 64 = 32 g Answer is- Mass of SO2 produced = 32 g.
Question 5.
Explain
(A) The need of the term average atomic mass.
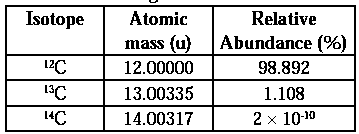
Average atomic mass : An element may have different isotopes i.e., atoms having same number of electrons and protons but different number of neutrons. The isotopes have different atomic masses and they exist in different proportion or abundance. The atomic mass of an element is the weighted average of atomic masses of its isotopes. This is called average atomic mass, Isotopes with relative abundances and atomic masses as shown against each of them. From the above data, the average atomic mass of carbon = (12 u) (98.892/100) + (13.00335 u) (1.108/100) + (14.00317) (2 × 10-10/100) = 12.011 u Similarly, average atomic masses for other elements can be calculated.
(B) Molar mass.
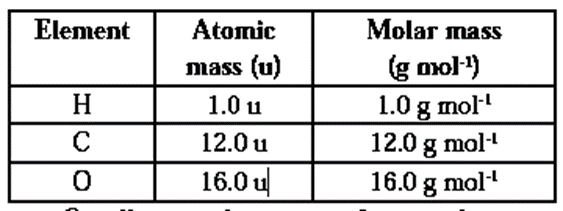
Molar Mass :The mass of one mole of a substance (element / compound) in grams is called its molar mass. The molar mass of any element in grams is numerically equal to atomic mass of that element in u. Similarly molar mass of any substance, existing as polyatomic molecule, in grams is numerically equal to its molecular mass or formula mass in u.

(C) Mole concept.
Mole : One mole of a substance is defined as the amount of a substance that contains the number of particles like atoms, molecules, ions or electrons equal to the number of carbon atoms present in 0.012 kg of Carbon-12 i.e., 6.0221367 x 1023 particles. One mole of a substance contains 6.022 x 1023 molecules while one mole (or one gram atom) of an element contains 6.0221367 x 1023 atoms.
(D) Formula mass with an example.
Formula mass : It is the sum of atomic masses of the atoms present in the formula of the substance. Explanation : Consider the formula mass of NaNO3. Formula mass of NaNO3 = average atomic mass of Na + average atomic mass of N + 3 x average atomic mass of O =23 u + 14 u + 3 x 16u = 85u
(E) Molar volume of gas.
Molar volume of a gas : The volume of one mole of a gas is called molar volume. At STP condition (0°C and 1 atm), the molar volume of any gas is 22.4 dm3. Volume of one mole of a gas at STP : One mole of any gas occupies a volume of 22.4 dm3 at standard temperature and pressure condition. Number of moles of a gas (n) = \(\frac{\text{Volume of the gas at STP}}{\text{Molar volume of gas}}\) = \(\frac{\text{Volume of the gas at STP}}{22.4}\) ∴ Number of molecules = number of moles x NA = number of moles x 6.022 x 1023 mol-1
(F) Types of matter (on the basis of chemical composition).
A matter may be an element, like Cu, Ag, etc. or may be a compound like NaCl, H2O, etc.
PDF : Class-11-Chemistry-Chapter-1-Some Basic Concepts of Chemistry- Notes
PDF : Class-11-Chemistry-Chapter-1-Some Basic Concepts of Chemistry-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Next Chapter : Chapter-2-Introduction to Analytical Chemistry – Online Solution
We reply to valid query.