Basic Principles of Organic Chemistry
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -14
Notes Part-1
|
Topics to be Learn : Part-1
|
Introduction :
- Only carbon is able to form an immense array of compounds, ranging from methane having one carbon atom to deoxyribonucleic acid (DNA) with billions of carbon atoms.
- Crude oil is a complex mixture of compounds called hydrocarbons.
Structural Representation of organic molecules :
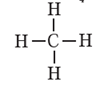
In a molecule's structural formula, each constituent atom is represented by its symbol, and any covalent bonds between atoms that are mutually bound are shown by a dash.
Example : Structural formula of CH4
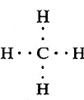
Electron-dot structure : In the electron-dot structure of a molecule, the valence electrons of all the atoms are shown as dots around them. Two dots shared between two atoms indicate one covalent bond.
Example : Electron dot structure of methane
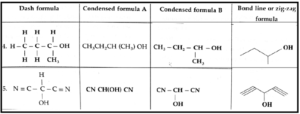
Condensed formula :
The complete structural formula is simplified with hiding of some or all the covalent bonds and indicating the number of identical groups attached to an atom by a subscript. The resulting formula of a compound is known as condensed formula.
Example : Condensed formula of ethane can be written as CH3 — CH3 or CH3CH3
Bond line formula or zig-zag formula :
- In this representation, symbols of carbon and hydrogen atoms of a molecule are not written. The lines representing carbon-carbon bonds are drawn in a zig-zag manner.
- The terminals of the zig-zag line represent methyl groups. The intersection of lines denotes a carbon atom bonded to appropriate number of hydrogen which satisfy the tetravalency of carbon atoms.
- For example, propane is represented by the zig-zag formula

- However, the heteroatoms or hydrogen atoms bonded to hetero atoms are written symbolically. The bond line formula of C2H5OH is
 OH
OH
Representation of structural formula :

Drawing the molecules in three dimensions :
Four different methods are used to represent three dimensional molecules on plane paper.
(i) Wedge formula : The three dimensional (3-D) view of a molecule can be represented on plane paper by representing the single bonds using,
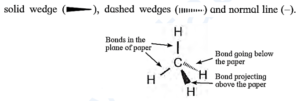
A solid wedge is represented by a bond projecting up from the paper towards the
reader. A dashed wedge is represented by a bond going backward, below the paper away from the reader. Normal lines indicate bonds in the plane of the paper. See Fig. (for convenience solid and dashed wedge can be replaced by solid/bold and dashed lines.)
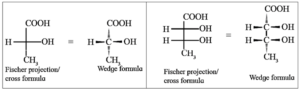
(ii) Fischer projection formula or cross formula :
The three dimensional (3—D) view of a molecule is presented on plane of paper. A Fischer projection formula can be drawn by visualizing the main carbon chain vertical in the molecule. Each carbon on the vertical chain is represented by a cross.
(iii) Norman projection formula: The three dimensional (3—D) molecule on the plane of the B paper is drawn by visualizing the molecule by holding the central C —C bond perpendicular to the paper. In the Newman projections, the rear carbon atom cannot be seen, being the shadow of the front carbon. A convention is followed to represent the front carbon by a point and the rear carbon by a circle around it.
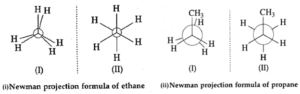
The remaining three bonds at each of these two carbons are drawn like spokes of a wheel. Conventionally three bonds at the front carbon are shown to emerge from the centre of the circle (which is the front carbon) and three bonds at the rear carbon are shown to emerge from the circumference of the circle.
(iv) Sawhorse or andiron or perspective formula : The projection is taken by holding the central C-C bond slightly inclined to the plane of the paper.
- The lower end of the line represents the front carbn and the upper end the rear carbon.
- The remaining three bonds at the two carbons are shown to radiate from the respective carbons.
- (As the central C-C bond is drawn rather elongated the bonds radiating from the front and rear carbons do not intermingle.)
Examples :
(i) Two sawhorse (i.e. andiron or perspective) formulae for ethane :
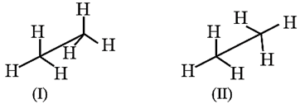
(ii) Two sawhorse formulae for the propane molecule :

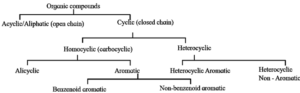
Classification of organic compounds :
Organic compounds are broadly classified in two ways, on the basis of (i) carbon skeleton and (ii) functional group.
Classification based on carbon skeleton :
(1) Aliphatic or Acyclic or open chain compounds :
The organic compounds in which carbon atoms are joined to form an open chain are called open chain compounds or aliphatic or acyclic compounds.
Example : n-butane CH3-CH2-CH2-CH3.
The aliphatic compounds may be
(a) Straight chain compounds : The chain of carbon atom is unbranched or straight.
Example : Propane CH3-CH2-CH3,
(b) Branched chain compounds : The chain of carbon atoms has branching (side chain).
Example :

(2) Cyclic or closed chain compounds : The organic compounds in which carbon atoms are joined to form one or more rings are called closed chain or cyclic or ring compounds. The cyclic compounds are further divided into
- Homocyclic or carbocyclic compounds and
- Heterocyclic compounds
(a) Homocyclic or carbocyclic : In these cyclic organic compounds the ring is made up of carbon atoms only. They are further divided into
- Alicyclic or open chain compounds
- Aromatic compounds.
(i) Alicyclic or gpen chain compounds : These compounds resemble aliphatic compounds in some properties. In these alicyclic or open chain compounds, carbon atoms are joined by single bonds. These are straight chain or branched compounds.
Examples :

(i) Aromatic compounds : These compounds contain at least one aromatic ring resembling benzene (with alternate single and double bonds) in their chemical properties.
Examples :
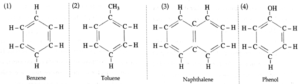
- Aromatic compounds are further classified as benzenoid and non-benzenoid compounds. Benzenoid compounds contain at least one benzene ring in the structure.
Examples :

- Non-benzenoid compounds contain an aromatic ring, other than benzene.
Examples :
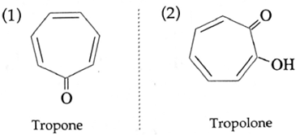
(b) Heterocyclic compounds :
The cyclic organic compounds which contain one or more hetero atom (other than carbon atom) in the ring are called heterocyclic organic compounds. The hetero atom may be Oxygen, Nitrogen or Sulphur.
Heterocyclic compounds are further divided into
- Heterocyclic non-aromatic compounds
- Heterocyclic aromatic compounds.
(i) Heterocyclic non-aromatic compounds:
The alicyclic compounds which contain at least one hetero atom in the ring (of carbon atoms) are called Heterocyclic non-aromatic compounds.
Examples :
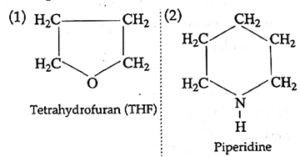
(ii) Heterocyclic aromatic compounds :
The aromatic compounds which contain at least one hetero atom in the ring (of carbon atoms) are called Heterocyclic aromatic compounds.
Examples :

Classification based on functional group :
- A part of an organic molecule which undergoes change as a result of a reaction is called functional group.
- The second method of classification of organic compounds is based on the nature of functional group present.
- The resulting individual class is called a family named after the constituent functional group. For example: family of alcohols, family of halogen derivatives.
Below table shows some common functional groups with their names, bond structures and examples.
Table : Functional groups in organic compounds :
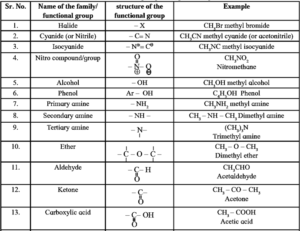
Homologous series :
A series of compounds of the same family in which each member has the same type of carbon skeleton and functional group, and differs from the next member by a constant difference of one methylene group (—CH2—) in its molecular and structural formula is called as homologous series. An individual member of a homologous series is called homologue.
Characteristics of homologous series :
- Each member of the homologous series can be represented by a general formula.
- Homologues have same functional groups and similar chemical properties.
- Two successive homologues differ by one —CH2 (methylene) unit and hence molecular weight of each successive member differs by 14.
- All the members of a homologous series can be prepared by similar methods.
- Physical properties (like melting point, boiling point, density) of the homologues show a gradual change with increase in the molecular weight of the member.
- Example : Homologous series of alcohols have general formula CnH2n+1
Homologous series of straight chain aldehydes : The members of a particular homologous series possess similar chemical properties those very gradually in their physical properties namely, melting point, boiling point, density, solubility, etc. Table illustrates this with the help of homologous series of straight chain aldehydes having general formula CnH2nO .
Table : Homologous series of straight chain aldehydes :
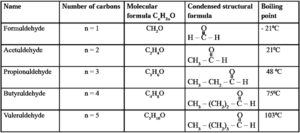
1°, 2°, 3° and 4° carbon atom :
The saturated (sp3) carbons in a molecule are labelled as primary, secondary, tertiary and quaternary in accordance with the number of other carbons bonded to it by single bonds.
- Primary carbon (10) is bonded to only one other carbon. For example: both the carbons in ethane CH3-CH3.
- Secondary carbon (20) is bonded to two other carbons. For example: the middle carbon in propane CH3-CH2-CH3
- Tertiary Carbon (30) is bonded to three other carbons. For example: the middle carbon in isobutene CH3CH(CH3)2.
- Quaternary Carbon (40) in bonded to four other carbons. For example: the middle carbon in neo-pentane C(CH3)4
Nomenclature of organic compounds
Common/trivial names :
- Common name of a compound usually has some history behind and usually accepted on account of its long usage.
- Though a systematic method of nomenclature was developed later, common names are still useful and in many cases cannot be avoided, particularly for commonly used commercial organic compounds.
Stem names of certain common organic compounds are given in below Table :

IUPAC Nomenclature :
The IUPAC names of a compound are obtained by modifying the name of its parent hydrocarbon further incorporating names of the branches and functional groups as prefix and suffix. For doing this, certain rules were formulated by IUPAC.
IUPAC names of straight chain alkanes :
The IUPAC name of a straight chain alkane is derived from the number of carbon atoms it contains.
Table : IUPAC names of the first twenty alkanes :
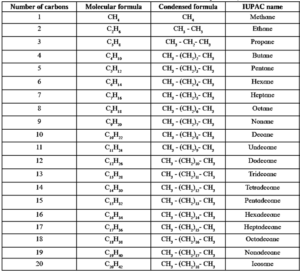
IUPAC names of branched saturated hydrocarbons :
The branches or the side chains in saturated hydrocarbons are alkyl groups or alkyl substituents.
Alkyl groups : A straight chain alkyl group is generated by removing one hydrogen atom from the terminal carbon of an alkane molecule and is named by replacing 'ane' of the alkane by 'yl'.
Table : Straight chain alkyl groups :
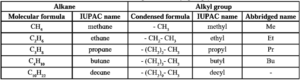
A branched chain alkyl group is obtained by removing a hydrogen atom from any one of the nonterminal carbons of an alkane or any hydrogen atom from a branched alkane.
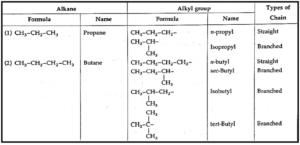
Note : n-alkyl groups are derived by removing a hydrogen atom from terminal or primary carbon atom. While branched alkyl groups are derived by removing a hydrogen atom from an internal (non-terminal) carbon atom.
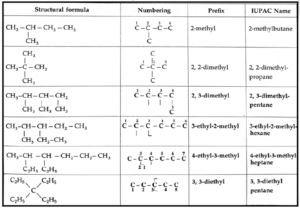
Rules for IUPAC nomenclature of branched saturated hydrocarbons :
(i) Select the longest continuous chain of carbon atoms to be called the parent chain. All other carbon atoms not included in this chain constitute side chains or branches or alkyl substitutents.
(ii) If two chains of equal length are present, the one with maximum number of substituents (or side chains) is selected as the parent chain.
(iii) The parent chain is numbered from one end to the other to locate the position, called locant number of the alkyl substitutent. The numbering is done in that direction which will result in lowest possible locant numbers .
(iv) Assign a number (locant) to each substituent according to its point of branching on the parent chain.
(v) If there are two substituents present on the same carbon atom, then the number of locant is repeated for each substituent.
(vi) If two or more different substituents are present, their names are entered in alphabetical order.
(vii) If two or more identical substituents are present, prefixes di, tri, tetra, etc. are used. [Prefixes are not considered while writing the names of substituents in alphabetical order.]
(viii) If two different substituents are located at equivalent positions from the two ends of the parent chain, then numbering of the carbon atoms of the chain is done such that the substituent which comes first in alphabetical order should get the lower number (locant).
(ix) If two chains of equal size are present (if two or more carbon chains compete for selection) then the carbon chain is selected which bears maximum number of substituents (side chains).
(x) Numbers are separated using a comma (,).
(xi) A number and a word are separated using a hyphen (-).
(xii) The IUPAC name of an alkane is written as one word.
(xiii) If the substituent (side chain) is further branched, it is named as substituted alkyl group, by numbering the carbon atom of this group attached to the parent chain as 1. The name of such a substituent is enclosed in brackets (to avoid confusion with the numbering of the parent chain).
IUPAC nomenclature of unsaturated hydrocarbons (Alkenes and Alkynes) :
While writing IUPAC names of alkenes and alkynes following rules are to be followed in addition to rules already discussed.
(i) The longest continuous chain must include carbon-carbon multiple bond. Thus the longest continuous chains in I and II contain four and six carbons, respectively.
(ii) Numbering of this chain must be done such that carbon-carbon multiple bond has the lowest possible locant number.
(iii) The ending 'ane' of alkane is replaced by 'ene' for an alkene and 'yne' for an alkyne.
(iv) Position of carbon atom from which multiple bond starts is indicated by smaller locant number of two multiply bonded carbons before the ending 'ene' or 'yne'
(v) If the multiple bond is equidistant from both the ends of a selected chain then carbon atoms are numbered from that end which is nearer to first branching.
(vi) If the parent chain contains two double bonds or two triple bonds, then it is named as diene or diyne. In all these cases 'a' of 'ane' (alkane) is retained .
(vii) If the parent chain contains both double and triple bond, then carbon atoms are numbered from that end where multiple bond is nearer. Such systems are named by putting 'en' ending first followed by 'yne'. The number indicating the location of multiple bond is placed before the name.
(viii) If there is a tie between a double bond and a triple bond, the double bond gets the lower number.
IUPAC Names of simple monocyclic hydrocarbons :
(i) A saturated monocyclic hydrocarbon is named by attaching prefix 'cyclo' to the name of the corresponding open chain alkane.
(ii) An unsaturated monocyclic hydrocarbon is named by substituting 'ene', 'yne' etc. for 'ane' in the name of corresponding cycloalkane.
(iii) If side chains are present then the above discussed rules are applied. Numbering of the ring carbon is started from a side chain.
(iv) If side chains are present then the above discussed rules are applied. Numbering of the ring carbon is started from a side chain.
Examples :
IUPAC nomenclature of compounds containing one or more functional groups :
(i) The functional group in the molecule is first identified and accordingly appropriate suffix is used.
(ii) The longest continuous chain of carbon atoms having functional group is selected.
(iii) The longest carbon chain is numbered from that end which is nearest to the functional group.
(iv) Using the appropriate number of the functional group, the complete name is written.
(v) The functional groups -F (fiuoro), -Cl (chloro), -Br (bromo), -I (iodo), -NO2 (nitro), -OR (alkoxy), -R (Alkyl), -Ar (aryl) are always prefix substituents.
(vi) In case of polyfunctional compounds, one of the functional groups is chosen as the principal functional group and the compound is named on that basis.
(vii) The principal functional group is decided on the basis of the following order of priority :
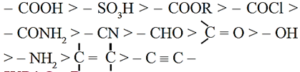
(viii) If two functional groups of the same type are present they are indicated by using di, tri, etc. before the class name suffix. Full name of the parent alkane is written before the class name suffix.
(ix) In case compounds have more than one, double or triple bond, the ending -ne of the parent alkane is dropped.
Table : Modified names of functional groups as in IUPAC nomenclature :
(a) Functional groups appearing prefix and suffix :

(b) Functional groups appearing only as prefix :

IUPAC nomenclature of substituted benzene :
Monosubstituted benzene :
- The IUPAC name of a monosubstituted benzene is obtained by placing the name of substituent as prefix to the parent compound benzene.
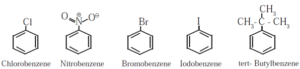
- If the alkyl substituent is larger than benzene ring (7 or more carbon atoms) the compound is named as phenyl-substituted alkane.

- Benzene ring can as well be considered as substituent when it is attached to an alkane with a functional group.
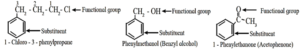
Disubstituted benzene derivatives :
- The prefixes ortho (o-), meta (m-) or para (p-) are used as common names of the three possible isomers of disubstituted benzene derivatives [IUPAC system, uses numbering instead of prefixes, o-, m-, or p-]
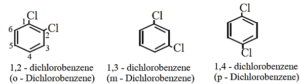
- If two substituents are different, then they enter in alphabetical order.
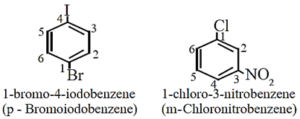
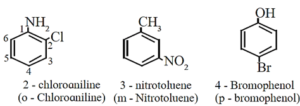
- If one of the two groups gives special name to the molecule then the compound is named as derivative of that special compound.
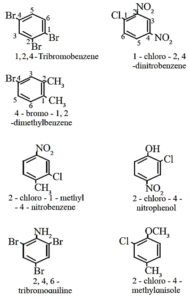
Trisubstituted benzene derivatives :
- The alphabetical order and lowest locant rule is followed, in some cases, common name of benzene derivatives is taken as parent compound.
- For example, if more than two substituents are attached to benzene ring, numbers are used to indicate their relative positions.
PDF : Class-11-Chemistry-Chapter-14-Basic Principles of Organic Chemistry-Text Book
PDF : Class-11-Chemistry-Chapter-14-Basic Principles of Organic Chemistry- Notes
PDF : Class-11-Chemistry-Chapter-14-Basic Principles of Organic Chemistry-Solution
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-13-Nuclear Chemistry and Radioactivity – Online Notes
Next Chapter : Chapter-15-Hydrocarbons – Online Notes