|
Topics to be Learn : Part-1
- Introduction
- Biomolecules in the cell
- Carbohydrates
- Lipids
- Proteins
Topics to be Learn : Part-2
- Nucleic Acids
- Enzymes
- Concept of Metabolism
|
Introduction :
Cell components and functions ::
Three main components of any cell :
- Cell membrane : Cell membrane separates the cytoplasmic contents from external environment.
- Cytoplasm: Site for metabolic activities and organelles.
- Nucleus: It is the control center of the cell. Genetic material is present in the nucleus.
The components present in both plant and animal cells :
- Endoplasmic reticulum: It produces, processes and transports proteins and lipids.
- Ribosomes: Ribosome is the site for protein synthesis. -
- Golgi apparatus: It is involved in modifying, sorting and packing of proteins for secretion. It also transports lipids around the cell.
- Lysosomes: It is involved in digestion of Wom out organelles and waste removal.
- Mitochondria: It is responsible for production of energy.
- Vacuoles: It has various functions like storage, waste disposal, protection and growth.
The components present in plant cell and not in animal cell:
- Cell wall: It provides strength and support to the cell. _
- Plastids: They are responsible for production and storage of food. It also contains photosynthetic pigments (Chloroplasts).
The components present in animal cell and not in plant cell:
- Cilia and flagella: Help in motility.
Classification of living organisms :
- Living organisms are classified as unicellular (consisting of single cell) : Examples : bacteria, yeast.
- Multicellular (having many cells) : Examples : plants, animals.
Biochemistry : Biochemistry is biological chemistry that provides us the idea of the chemistry of living organisms and molecular basis for changes taking place in plants, animals and microbial cells.
- It develops the foundation for understanding all biological processes and communication within and between cells as well as chemical basis of inheritance and diseases in animals and plants.

Chemical analysis of living organisms :
Chemical analysis of living organisms : Chemical analysis of all living organisms indicates the presence of the most common elements like carbon, hydrogen, nitrogen, oxygen, sulphur, calcium, phosphorus, magnesium and others with their respective content per unit mass of a living tissue.
- To identify the chemical compounds present in the living tissue chemical analysis is performed.
- Various separation techniques are used to extract, isolate and purify organs compounds.
- The analysis of chemical compounds gives an idea about the molecular formula and structure of the compound.
- To analyse various biomolecules in a living tissue: Living tissue (vegetable or piece of liver) is ground in trichloroacetic acid using mortar and pestle. —>The thick slurry is filtered through cotton or cheesecloth.—> From the obtained two fractions, one is called as filtrate (the acid soluble fraction) and other fraction is called retentate (the acid-insoluble fraction).
- The acid-soluble pool contains thousands of organic compounds.
- All the carbon compounds obtained from living tissues are called ‘Biomolecules.
- Along with organic elements and compounds, living organisms also show presence of inorganic elements and compounds.
[collapse]
Basic macromolecules present in the living organisms :
Polysaccharldes (carbohydrate) polymer of monosaccharide, polypeptides (proteins) polymer of amino acids and polynucleotides (nucleic acids) polymer of nucleotides are the three basic macromolecule present in the living organisms.
Biomolecules in the cell :
Carbohydrates : Carbohydrates mean ‘hydrates of carbon’. They are also called saccharides.
- They are biomolecules made from just three elements: carbon, hydrogen and oxygen with the general formula (CH2O)n.
- They contain hydrogen and oxygen in the same ratio as in water (2:1).
- Carbohydrates can be broken down (oxidized) to release energy.
- Carbohydrates provide energy for metabolism.
- Glucose is the main substrate for ATP synthesis.
- Lactose, a disaccharide present in the milk provides energy to babies.
- Polysaccharide serves as a structural component of cell membrane, cell wall and reserved food as starch and glycogen.
Classes of carbohydrates :
Based on number of sugar units, carbohydrates are classified into three types namely, monosaccharides, disaccharides and polysaccharides.
Monosaccharides :
Monosaccharides:
- Monosaccharides are the simplest sugars having crystalline structure, sweet taste and soluble in water.
- They cannot be further hydrolyzed into smaller molecules.
- They are the building blocks or monomers of complex carbohydrates.
- They have the general molecular formula (CH2O) n, where n can be 3, 4, 5, 6 and 7.
- They can be classified as triose, tetrose, pentose, etc.
- All monosaccharides are reducing sugars due to presence of free aldehyde or ketone group. These sugars reduce the Benedict's reagent (Cu2+ to Cu+) since they are capable of transferring hydrogens (electrons) to other compounds, a process called reduction.
- Monosaccharides containing the aldehyde (—CHO) group are classified as aldoses e.g. glucose, xylose, and those with a ketone(—C=O) group are classified as ketoses. E.g. ribulose, fructose.
Properties of some Monosaccharide molecules : It depend on its length, branching, folding and coiling.
Glucose: .
- It is the most important fuel in living cells.
- Its concentration in the human blood is about 90mg per 100ml of blood.
- The small size and solubility in water of glucose molecules allows them to pass through the cell membrane into the cell.
- Energy is released when the molecules are metabolized by cellular respiration.
Galactose:
- It looks very similar to glucose molecules.
- They can also exist in on and B forms. .
- Galactose react with glucose to form the disaccharide lactose.
- However, glucose and galactose cannot be easily converted into one another.
- Galactose cannot play the same role in respiration as glucose.
Fructose:
- It is the fruit sugar and chemically it is ketohexose but it has a five-atom ring rather than a six-atom ring.
- Fructose reacts with glucose to form the sucrose, a disaccharide.
[collapse]
Disaccharides :
Disaccharides:
- Disaccharide is formed when two monosaccharide react by condensation reaction releasing a water molecule. This process requires energy.
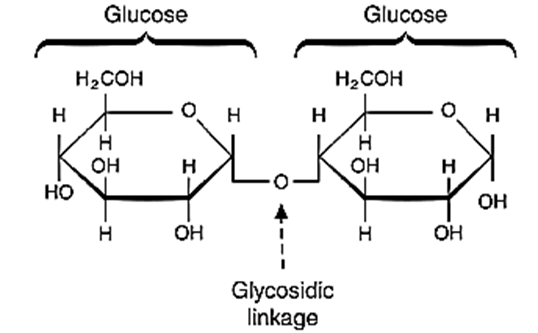
- A glycosidic bond forms and holds the two monosaccharide units together.
- Sucrose, lactose and maltose are examples of disaccharides.
- Sucrose is a non-reducing sugar since it lacks free aldehyde or ketone group.
- Lactose and maltose are reducing sugars.
- Lactose also exists in beta form, which is made from B-galactose and B-glucose.
- Disaccharides are soluble in water, but they are too big to pass through the cell membrane by diffusion. They are broken down in the small intestine during digestion. Thus, formed monosaccharides then pass into the blood and through cell membranes into the cells C12H22O11 + H2O \(\underrightarrow {i}\) C6H12O6+ C6H12O6

[collapse]
Polysaccharides :
Polysaccharides:
- Monosaccharides can undergo a series of condensation reactions, adding one unit after the other to the chain till a very large molecule (polysaccharide) is formed. This is called polymerization.
- Polysaccharides are broken down by hydrolysis into monosaccharides.
- Polysaccharides are too big to escape from the cell
- The properties of a polysaccharide molecule depends on its length, branching, folding and coiling.
- Examples: Starch, glycogen, cellulose.
- The exoskeleton of insects is made up of chitin. This is a polysaccharide
The polysaccharides that take part in the forming the structural framework of the cell wall of plants and skeleton of animals.
Names of structural polysaccharides : Arabinoxylans, cellulose, chitin, pectin.
- Arabinoxylans: Found in primary and secondary cell walls of plants.
- Cellulose: Main component of cell wall in plants.
- Chitin: Main component of Exoskeleton. '
- Pectin: Present in primary cell walls and in non-woody parts of terrestrial plants.
There are two types of polysaccharides:
- Homopolysaccharides: lt contains same type of monosaccharides. E.g. Starch, glycogen, cellulose
- Heteropolysaccharides: It contains two or more different monosaccharides. E.g. Hyaluronic acid, heparin hemicellulose.
[collapse]
Properties of some Polysaccharide molecules : Properties of Polysaccharide molecules depend on its length, branching, folding and coiling.
Starch :
- Natural Sources: Cereals (wheat, maize, rice), root vegetables (potato, cassava etc.)
- Structural units: Starch consist of two types of molecules — Amylose and amylopectin.
- Functions: It is used as thickener, water, emulsion stabilizer and gelling agent.
Properties of Starch :
- Starch is a stored food in the plants.
- Starch contains two types of glucose polymer: amylose and amylopectin
- Both are made from α-glucose.
- Amylose is an unbranched polymer of α-glucose.
- The molecules coil into a helical structure.
- It forms a colloidal suspension in hot water.
- Amylopectin is a branched polymer of α-glucose.
- It is completely insoluble in water.
Glycogen:
- It is amylopectin with very short distances between the branching side-chains
- Glycogen is stored in animal body particularly in liver and muscles from where it is hydrolyzed as per need to produce glucose.
Cellulose:
- Natural sources: Plant fibers (cotton, flax, hemp, jute, etc.), wood.
- Structural units: It is made from β-glucose molecules.
- Functions: It helps plants to remain stiff and strong. It is important in diet as a source of fibre. It is used to make paper and clothes.
Properties of Cellulose :
- It is a polymer made from β-glucose molecules and the polymer molecules are ‘straight’.
- Cellulose serves to form the cell walls in plant cells.
- These are much tougher than cell membranes.
- This touglmess is due to the arrangement of glucose units in the polymer chain and the hydrogen-bonding between neighbouring chains.
Oligosaccharides :
Oligosaccharides: A carbohydrate polymer comprising of two to six monosaccharide molecules is called oligosaccharide. They are linked together by glycosidic bond. They are classified on the basis of monosaccharide units:
- Disaccharides: These are the sugars containing two monosaccharide units and can be further hydrolysed into smaller components. E.g.: Sucrose, maltose, lactose, etc.
- Trisaccharidcs: These contain three monomers. E.g. Raffinose.
- Tetrasaccharides: These contain four monomers. E.g.: Stachyose.
[collapse]
Glycosidic bond: Glycosidic bond is a covalent bond that forms a linkage between two monosaccharides dehydration reaction.
- It is formed when a hydroxyl group of one sugar reacts with the anomeric carbon of the other.
- Glycosidic bonds are readily hydrolyzed by acid but resist cleavage by base
There are two types of glycosidic bonds: α-glycosidic bond and β-glycosidic bond.
Classification of Carbohydrates :
|
Classification of Carbohydrates
|
| Monosaccharides
(Simple sugars)
1. Triose-3carbons (e.g. Glyceraldehyde)
2. Tetrose-4 carbons (e.g. Erythrose)
3. Pentose-5 carbons (e.g. Ribose in RNA and deoxyribose in DNA)
4. Hexose- 6 carbons (e.g. Glucose- blood sugar, Fructose-fruit sugar and Galactose-product of lactose)
5. Heptose-7 carbons (e.g. Sedoheptulose)
|
Disaccharides
(Two monosaccharides)
1. Sucrose (cane sugar) on hydrolysis, it produces Glucose and Fructose
2. Lactose (milk sugar) on hydrolysis, it produces Glucose and Galactose
3. Maltose (malt sugar) on hydrolysis, it produces two units of Glucose
|
Polysaccharides
(Polymer of monosaccharides)
1. Homopolysaccharides:
polymer of one type of monosaccharides
e.g. Starch - plant storage molecule
e.g. Cellulose - cell wall component
e.g. Glycogen - animal storage molecule
2. Heteropolysaccharides:
polymer of different types of monosaccharides e.g. Hyaluronic acid, heparin, blood group substances, chondroitin sulphate
|
[collapse]
Lipids:
- Lipids are a group of heterogeneous compounds like fats, oils, steroids, waxes, etc.
- They are macro-biomolecules.
- These are group of substances with greasy consistency with long hydrocarbon chain containing carbon, hydrogen and oxygen.
- Lipid is a broader term used for fatty acids and their derivatives.
- They are soluble in organic solvents (non-polar solvents).
Fatty acids are organic acids which are composed of hydrocarbon chain ending in carboxyl group (-COOH). Fatty acids are classified into Saturated and Unsaturated fatty acids.
Saturated and Unsaturated fatty acids :
Saturated fatty acids: They contain single chain of carbon atoms with single bonds.
- E.g. Palmitic acid, stearic acid
Unsaturated fatty acids: They contain one or more double bonds between the carbon atoms of the hydrocarbon chain. Oleic acid found in nearly all fats and linoleic acid found in many seed oils are examples of unsaturated fatty acids.
- Simple lipids: These are esters of fatty acids with various alcohols. E.g. Fats, wax.
- Compound lipids: These are ester of fatty acids containing other groups like phosphate (Phospholipids), sugar (glycolipids), etc. E.g. Lecithin
- Sterols: They are derived lipids. They are composed of fused hydrocarbon rings (steroid nucleus) and a long hydrocarbon side chain. E.g. Cholesterol, phytogsterols.

[collapse]
Types of lipids and their biological significance :
Lipids are classified into three main types:
(i) Simple lipids :
(i) Simple lipids :
- These are esters of fatty acids with various alcohols. Fats and waxes are simple lipids.
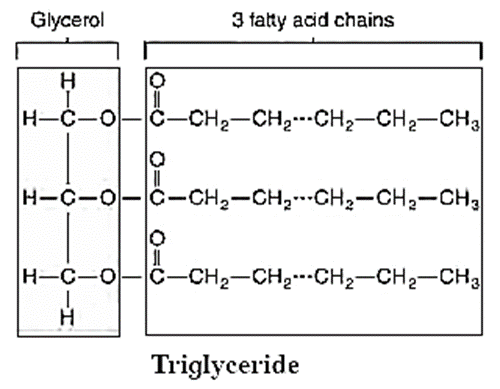
- Fats are esters of fatty acids with glycerol (CH2OH-CHOH-CH2OH).
- Triglycerides are three molecules of fatty acids and one molecule of glycerol.
- Unsaturated fats are liquid at room temperature and are called oils. Unsaturated fatty acids are hydrogenated to produce fats e.g. Vanaspati ghee.

Biological significance:
- Fats are a nutritional source with high calorific value and they act as reserved food materials.
- In plants, fat is stored in seeds to nourish embryo during germination.
- In animals, fat is stored in the adipocytes of the adipose tissue.
- Fats deposited in subcutaneous tissue act as an insulator and minimize loss of body heat.
- Fats deposited around the internal organs act as cushions to absorb mechanical shocks.
- Wax is another example of simple lipid. They are esters of long chain fatty acids with long chain alcohols.
- They are most abundant in the blood, the gonads and the sebaceous glands of the skin.
- Waxes are not as readily hydrolyzed as fats.
- They are solid at ordinary temperature.
- Waxes form water insoluble coating on hair and skin in animals, waxes form an outer coating on stems, leaves and fruits.
[collapse]
(ii) Compound lipids :
(ii) Compound lipids:
- These are ester of fatty acids containing other groups like phosphate (Phospholipids), sugar (glycolipids), etc.
- They contain a molecule of glycerol, two molecules of fatty acids and a phosphate group or simple sugar.
- Some phospholipids such as lecithin also have a nitrogenous compound attached to the phosphate group.
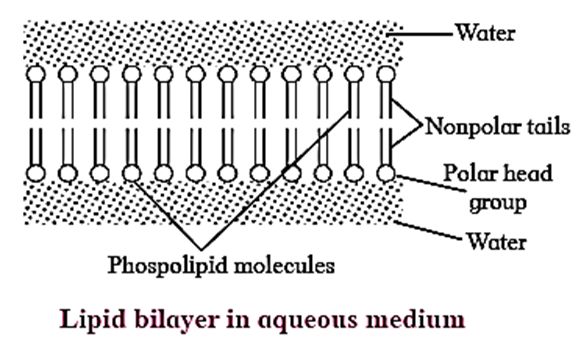
- Phospholipids have both hydrophilic polar groups (phosphate and nitrogenous group) and hydrophobic non-polar groups (hydrocarbon chains of fatty acids).
- Glycolipids contain glycerol, fatty acids, simple sugars such as galactose and nitrogenous base. They are also called cerebrosides.

Biological significance:
- Phospholipids contribute in the formation of cell membrane.
- Large amounts of glycolipids are found in the brain white matter and myelin sheath.
[collapse]
(iii) Derived Lipids :
(iii) Derived Lipids:
- They are composed of fused hydrocarbon rings (steroid nucleus) and a long hydrocarbon side chain_
- One of the most common sterols is cholesterol.
Biological significance: -
- It is widely distributed in all cells of the animal body, but particularly in nervous tissue.
- Cholesterol exists either free or as cholesterol ester.
- Adrenocorticoids, sex hormones (progesterone, testosterone) and vitamin D are synthesized from cholesterol.
- Cholesterol is not found in plants.
- Sterols exist phytosterols in plants.
- Yam Plant (Dioscorea) produces a steroid compound called diosgenin. It is used in the manufacture of antifertility pills. i.e. birth control pills.
[collapse]
Q. Why plant fats are liquid at room temperature while animal fats are solid?
Answer :
- Plant fats are unsaturated fatty acids, whereas animal fats are saturated fatty acids
- Fats having unsaturated fatty acids are liquid at room temperature.
- Saturated fatty acids are solid at room temperature.
- Hence, plant fats are liquid at room temperature, while animal fats are solid.
[collapse]
Q. Why do high cholesterol level in the blood cause heart diseases?
Answer :
- When there is high level of cholesterol in the blood, the cholesterol builds up on the walls of arteries causing a condition called atherosclerosis (a form of heart disease).
- Because of this the arteries are narrowed and the blood flow to the heart is slowed down.
- The blood carries oxygen to the heart, but because of this condition enough blood and oxygen does not reach to the heart and causes heart diseases.
- If the condition increases, the supply of oxygen and blood is completely cut off to the heart and this can lead to heart attack.
[collapse]
Q. Polyunsaturated fatty acids are believed to decrease blood cholesterol level. How?
Answer :
- The liver converts polyunsaturated fatty acids into ketones instead of cholesterol.
- Therefore, polyunsaturated fatty acids are transported directly to tissues for oxidation without leaving behind any lipoprotein in the form of cholesterol as it is seen in the case of saturated fatty acids.
- Thus, polyunsaturated fatty acids are believed to decrease blood cholesterol level.
[collapse]
Proteins :
Protein is the complex organic nitrogenous substances found in the cell of all animals and plants.
Characteristics :
Characteristics :
- Proteins are large molecules containing amino acid units ranging from 100 to 3000.
- Proteins have high molecular weights.
- They contain C, H, O and N. The presence of N distinguishes them from carbohydrates and lipids. Some proteins contain sulphur, while few proteins contain phosphorus also. Cells contain a large number of protiens.
- In proteins, amino acids are linked together by peptide bonds which join the carboxyl group of one amino acid residue to the amino group of another residue.
- A protein molecule consists of one or more polypeptide chains.
- Proteins can contain any or all of the 20 naturally occurring amino acid types.
- Proteins are extremely reactive and highly specific in behaviour.
- Proteins are amphoteric in nature i.e. they act as both acids and bases.
- The behaviour of proteins is strongly influenced by pH.
- Like amino acids, proteins are dipolar ions at the isoelectric point i.e. the sum of the positive charges is equal to the sum of the negative charges and the net charge is zero.
- The ionic groups of a protein are contributed by the side chains of the polyvalent amino acids.
- A protein consists of more basic amino acids such as lysine and arginine exist as a cation and behaves as a base at the physiological pH of 7.4. Such proteins are called basic proteins.
- Histones of nucleoproteins are basic proteins.
- A protein rich in acidic amino acids exists as an anion and behaves as an acid. Such proteins are called acidic proteins.
- Most of the blood proteins are acidic proteins.
[collapse]
Primary structure of protein :
The linear sequence of amino acids in polypeptide chain of a protein forms the primary structure of a protein.
Secondary structure of protein :
- There are two types of secondary structure of protein: α-helix and β-pleated sheets. The polypeptide chain is arranged in a spiral helix. These spiral helices are of two types: α-helix (right handed) and β-helix (left handed).
- This spiral configuration is held together by hydrogen bonds.
- The sequence of amino acids in the polypeptide chain determines the location of its bend or fold and the position of formation of hydrogen bonds between different portions of the chain or between different chains.
- Thus, peptide chains form an α-helix structure.
- Example of α-helix structure is keratin.
- In some proteins two or more peptide chains are linked together by intermolecular hydrogen bonds. Such structures are called β-pleated sheets.
- Example of β-pleated sheet is silk fibres.
- Due to formation of hydrogen bonds peptide chains assume 'a secondary structure.
Tertiary structure of protein: -
- In tertiary structure the peptide chains are much looped, twisted and folded back on themselves due to formation of disulphide bonds.
- Such loops and bends give the protein a tertiary structure.
- E.g. Myoglobin, enzymes.
Quaternary structure of protein:
- When a protein has more than two polypeptide subunits their arrangement in space is called quaternary structure.
- E.g. Haemoglobin
Peptide bond :
- The covalent bond that links the two amino acids is called a peptide bond.
- Peptide bond is formed by condensation reaction.
- Bond formed between two polypeptides is called Disulfide bond, amino acid Cysteine is involved in the formation of such bond.
Classes of proteins with their importance :
Classes of proteins with their importance :
On the basis of structure, proteins are classified into three categories:
(i) Simple proteins: -
- Simple proteins on hydrolysis yield only amino acids.
- These are soluble in one or more solvents.
- Simple proteins may be soluble in Water.
- Histones of nucleoproteins are soluble in water.
- Globular molecules of histones are not coagulated by heat.
- Albumins are also soluble in water but they get coagulated on heating.
- Albumins are widely distributed e. g. egg albumin, serum albumin and legumelin of pulses are albumins.
Importance: They are involved in structural components, they also act as a storage kind of protein.
Conjugated proteins:
- Conjugated proteins consist of a simple protein united with some non-protein substance.
- The non-protein group is called prosthetic group e. g. haemoglobin.
- Globin is the protein and the iron containing pigment haem is the prosthetic group.
- Similarly, nucleoproteins have nucleic acids as prosthetic group.
- Proteins are classified as glycoproteins and mucoproteins.
- Mucoproteins are carbohydrate-protein complexes e. g. mucin of saliva and heparin of blood.
- Lipoproteins are lipid-protein complexes e. g. conjugate protein found in brain, plasma membrane, milk etc.
Importance: They are involved in structural components of cell membranes and organelles. They also act as a transporter.
The conjugated protein functions in interaction with other chemical group whereas simple proteins contain only amino acids and no other chemical group attached to it.
Derived proteins:
- These proteins are not found in nature as such. ‘
- These proteins are derived from native protein molecules on hydrolysis.
- Metaproteins, peptones are derived proteins.
Importance: They act as a precursor for many molecules which are essential for life.
[collapse]
| The various health drinks like Boost, Horlicks, Bournvita, etc. available in the market contain essential vitamins and minerals It also contains high quality proteins which help to boost muscle mass, promote bone and muscle strength. These high-quality proteins are derived proteins. |
Biological functions of proteins :
Biological functions of proteins:
The biological importance of proteins is as follows:
- Membrane proteins: Cell membrane consists of proteins and lipids. All membrane bound cell organelles have lipids and proteins in their membranes.
- Enzymes: All enzymes are proteins. Enzymes may contain some other non-protein part also along with protein. e.g. Amylase.
- Hormones: Hormones are proteins. They play an important role in the regulation of metabolic reactions in the body. e.g. Insulin, thyroxine.
- Transport protein: Haemoglobin which is present in R.B.C. of man is a type of protein. It is useful for transportation of oxygen. .
- Contractile protein: Muscle fibres consist of proteins, which help in contraction. e.g. Myosin.
- Structural protein: These proteins form parts of cells or tissues. e.g. Keratin is present in hair and skin, while elastin is present in connective tissue.
- Defensive proteins: Useful for the protection of body. e.g. Immunoglobin, thrombin for clotting of blood.
[collapse]
| The proteins found in human beings and animals may be different from those of others because the ratio of amino acids present in the protein differs. |
Next Part ->>


We reply to valid query.