Elements of Groups 16, 17, and 18
Maharashtra Board-Class-12-Chemistry-Chapter-7
Notes-Part-2
Topics to be Learn : Part-2
|
Allotropy :
The property of some elements to exist in two or more different forms in the same physical state is called allotropy.
Property of allotropy in Group 16 elements :
- All the elements of group 16 exhibit allotropy.
- These elements exist in different allotropic modifications.
- Oxygen exists as O2 and ozone as O3.
- Sulphur exists as α-sulphur, β-sulphur, g-sulphur, homocyclic sulphur, plastic sulphur, etc. Rhombic sulphur (α sulphur) and mono-clinic sulphur (β sulphur) are the important allotropes. Both are non-metallic in nature.
- Selenium exists in two allotropic forms red (non-qxmetallic) and grey (metallic).
- Tellurium exists in two allotropic forms crystalline form and the amorphous form.
- Po has two fomqs namely α-form and β-form both being metallic.
| Know This :
Grey selenium allotrope of is a photoconductor used in photocells. The photocopying process :
Figure of photocopying process using Se is as shown below :
|
Allotropes of sulfur :
Sulfur exhibits numerous allotropic forms. However rhombic sulfur (α-sulfur) and monoclinic sulfur (β-sulfur) are the most important allotropes of sulfur.
Rhombic sulfur :
- Rhombic sulphur has orthorhombic crystals.
- This is the most stable form and common form of sulphur.
- It is pale yellow, having density 2.069 g/cm3 and melting point 385.8 K
- It is insoluble in water, but soluble in CS2
- It is stable below 369 K and transforms to β-sulphur above this temperature.
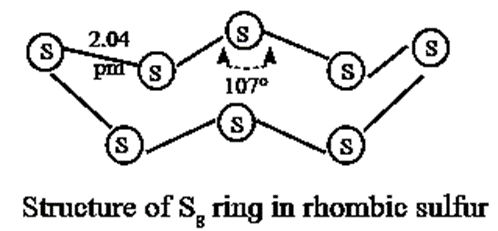
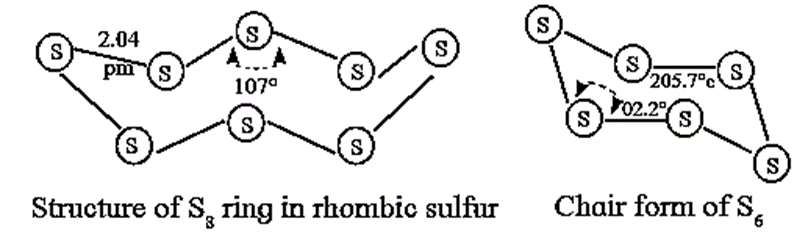
- It exists as S8 molecules with a structure of a puckered ring.
- It is obtained by the evaporation of roll sulphur is CS2.
Monoclinic sulfur :
- Monoclinic sulphur (β-sulphur or prismatic sulphur) has needle-shaped monoclinic crystals.
- It is bright yellow, having a density 1.989 g/cm3 and melting point 393 K.
- It is soluble in CS2.
- It is stable above 369 K and transforms into α-sulphur below this temperature.
- It exists as, S8 molecules with a structure of a puckered ring.
- It is prepared by melting rhombic sulphur and cooling it till a crust is formed. Two holes are pierced in the crust and the remaining liquid is poured to obtain needle-shaped crystals of monoclinic sulphur.
Remember :
- Several modifications of sulfur containing 6-20 sulfur atoms per ring, have been synsthesised. In the S8 molecule the ring is puckered and has a crown shape.
- In cyclo - S6, the ring adopts the chair form. At elevated temperature (~ 1000 K), S2 is the dominant species which like O2 is paramagnetic.
Oxoacids
Oxoacids of sulfur : Sulfur forms a number of oxoacids. Some of them are unstable and cannot be isolated. They are known to exist in aqueous solutions or in the form of their salts.
Some important oxoacids of sulfur and their structures are given below.

Oxoacids of halogens :
- Fluorine forms only one oxyacid namely, hypofluorous acid or fluoric acid HOP while other halogens form several oxyacids.
- Only four oxoacids have been isolated in pure form: hypofluorous acid (HOF), perchloric acid (HClO4), iodic acid (HIO3), metaperiodic acid (H2IO6).
- The others are stable only in aqueous solutions or in the form of their salts.
- Oxidising power of oxyacids of halogens decreases as the oxidation number of halogens increases.
HClO (1+) > HClO2 (3+) > HClO3 (5+) > HClO4 (7+)
(or HOCl > HOClO > HOClO2 > HOClO3 )
- The thermal stability of oxyacids of halogens increases with the increase in oxidation state of halogen. Hence the increasing order of thermal stability is,
HClO (1+) < HClO2 (3+) < HClO3 (5+) < HClO4 (7+)
- The acid strength of the halogen oxoacids increases with the increasing oxidation state of halogen. For example, acid strength increases from HClO, a weak acid (Ka = 3.5 × 10-8), to HClO4, a very strong acid (Ka>>1).
Oxoacids of halogens
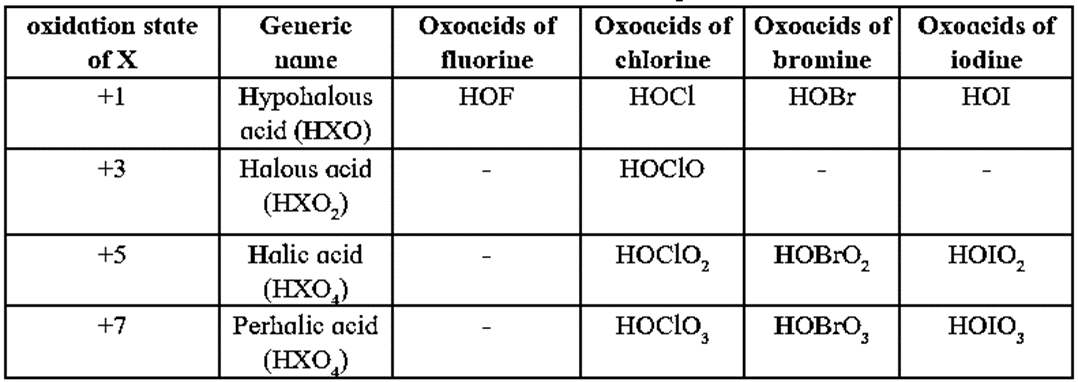
Structures of oxoacids of chlorine :

Oxygen and Compounds of oxygen :
Dioxygen :
Preparation of Dioxygen by Laboratory methods :
(i) By heating oxygen containing salts such as chlorates, nitrates and permanganates.
2KClO3(s) \(\frac{Heat}{MnO_2}\)-> 2KCl(s) + 3O2(g)
(ii) By thermal decomposition of oxides of metals.
2Ag2O(s) \(\underrightarrow{heat}\) 4Ag(s) + O2(g)
2HgO(s) \(\underrightarrow{heat}\) 2Hg(l) + O2(g)
2PbO2(s) \(\underrightarrow{heat}\) 2PbO(s) + O2(g)
(iii) By decomposition of hydrogen peroxide in presence of catalyst such as finely divided metals and manganese dioxide.
2H2O2(aq) (\frac{Heat}{MnO_2}\)-> 2H2O(l) + O2(g)
Dioxygen on a large scale or commercial scale is obtained by two following methods :
Preparation of Dioxygen from water (By electrolysis) : By electrolysis of acidified water, H2 gas is obtained at cathode and O2 is obtained at anode.
H2O(acidified) \(\underleftrightarrow{dilute\,\,H_2SO_4}\) H+ + OH−
At cathode : 2H+ + 2e− → H2(g)
At anode ; 2OH− → ½ O2(g) + H2 + 2e−
Preparation of Dioxygen from air (Industrial method) :
- Carbon dioxide and water vapour is removed fromair and the remaining gases are liquefied.
- O2 on large scale is obtained by fractional distillation of liquid air.
- On distillation, liquid dinitrogen having low boiling point distils out first leaving behind liquid dioxygen. Then liquid O2 is distilled out and separated.
Physical properties of Dioxygen :
- Dioxygen is colourless and odourless gas.
- Dioxygen is sparingly soluble in water, 30.8 cm3 of O2 dissolves in 1000 cm3 of water at 293 K. A small amount of dissolved dioxygen is sufficient to sustain marine and aquatic life.
- It liquifies at 90 K and freezes at 55 K.
- Oxygen has three stable isotopes 16O, 17O and 18
- Molecular oxygen, O2 exhibits paramagnetism.
Chemical Properties of Dioxygen :
(i) Reaction with metals : Dioxygen directly reacts with almost all metals except Au, Pt to form their oxides.
2Ca + O2 → 2CaO
4Al + 3O2 → 2Al2O3
(ii) Reaction with nonmetals : Dioxygen reacts with nonmetals (except noble gases) to form their oxides.
C + O2 → CO2
P4 + 5O2 → P4O10
(iii) Reaction with some compounds :
2ZnS + 3O2 \(\underrightarrow{Δ}\) 2ZnO + 2SO2
CH4 + 2O2 → CO2 + 2H2O
2SO2 + O2 \(\underrightarrow{V_2O_5}\) 2SO3
4HCl + O2 \(\underrightarrow{CuCl_2}\) 2Cl2 + 2H2O
Uses of Dioxygen :
- Dioxygen is important for respiration to sustain animal and aquatic life.
- It is used in the manufacture of steel.
- It is used in oxyacetylene flame for welding and cutting of metals.
- Oxygen cylinders are widely used in hospitals, high altitude flying and mountaineering.
- It is used in combustion of fuels; for example, hydrazine in liquid oxygen provides tremendous thrust (energy) in rockets.
Simple Oxides :
A binary compound of oxygen with another element is called an oxide.
Oxides can be classified into
- Acidic oxides, CO2, SO2, etc.
- Basic oxides, CaO, BaO, etc.
- Amphoteric oxides, Al2O3, ZnO, etc
- Neutral oxides, NO, N2O, CO, etc.
Acidic oxides :
- The oxide, which on reaction with water forms an acid or reacts with a base to give a salt is called an acidic oxide.
- It is formed by the combination of oxygen with non-metals. For example CO2, SO3, etc.
CO2 + H2O → H2CO3 (carbonic acid)
SO2 + H2O → H2SO3
SO3 + 2NaOH → Na2SO4 + H2O
- Generally, oxides of nonmetals are acidic oxides.
Basic oxides :
- The oxide, which on reaction with water forms a base or reacts with an acid to give a salt is called basic oxide.
- It is formed by the reaction of oxygen with highly electropositive metals.
- For example, Na2O, CaO, BaO, etc.
Na2O + H2O → 2NaOH
CaO + H2O → Ca(OH) 2
BaO + 2HCl → BaCl2 + H2O
- Basic oxides are generally ionic in nature.
Amphoteric oxides :
- The oxide, which shows both acidic and basic characteristics is called an amphoteric oxide.
- They are formed by the reaction of oxygen with elements which lie on the border of electropositive (metals) and electronegative (non-metals) nature.
- For example, Al2O3, ZnO, etc.
Al2O3(s) + 6HCl(aq) + 9H2O(l) → 2[Al(H2O)6]3+ + 6Cl−(aq)
Basic
Al2O3(s) + 6NaOH(aq) + 3H2O(l) → 2Na3[Al(OH)6](aq)
Acidic Base
Along the period, from left to right the nature of oxides changes from basic to amphoteric to acidic.
Neutral oxides : The oxide which behaves neither acidic nor basic is called a neutral oxide. For example, CO, NO, N2O.
Ozone :
- The stratospheric pool of ozone which is a layer above eax1h’s surface and protects from harmful high energetic ultraviolet (UV) rays is called ozone umbrella or ozonosphere. .
- Ozone (O3) is an allotrope of oxygen.
Ozone formation :
- In the atmosphere, ozone is naturally formed through photochemical reactions.
- Oxygen present in the lower mesosphere on absorption of solar radiations, is dissociated into two oxygen atoms which oxidise oxygen to ozone.
- One atomic oxygen combines with molecular oxygen to form O3.
O2 \(\underrightarrow{UV\,\,light}\) O + O
O2 + O → O3
Laboratory preparation of ozone :
- When a slow dry stream of oxygen is passed through a silent electric discharge, oxygen is converted into ozone (about 10%). The mixture is called ozonized oxygen.
3O2 \(\underrightarrow{Electric\,\,discharge}\) 2O3 …ΔH = +142kJ
- It is an endothermic reaction.
- Silent electric discharge prevents the decomposition of ozone.
Physical properties of ozone :
- Gaseous ozone is blue, liquid ozone is dark blue and solid ozone is violet black.
- It has pungent odour hence the name is ozone.
- At higher concentration (about 100 ppm), it is harmful and results into nausea and headache while in small concentration it is harmless.
- Ozone is thermodynamically less stable than oxygen.
- The decomposition of ozone (2O3 → 3O2) is exothermic (ΔH < O) and has ΔS > 0. Therefore ΔG < O and hence decomposition of O3 is spontaneous.
- Ozone is diamagnetic in nature.
Chemical Properties of Ozone :
(i) Oxidising property :
Ozone is a powerful oxidising agents as it easily decomposes to liberate nascent oxygen. (O3 → O2 + O).
Ozone oxidises lead sulfide to lead sulfate and iodide ions to iodine.
PbS(s) + 4O3(g) → PbSO4(s) + 4O2(g)
2KI(aq) + H2O(l) + O3(g) → 2KOH(aq) + I2(g) + O2(g)
Ozone oxidises nitrogen oxide and gives nitrogen dioxide.
NO(g) + O3(g) → NO2(g) + O2(g)
Hence the nitrogen oxide emitted from the exhaust systems of supersonic jet aeroplanes can bring forth depletion of ozone layer in the upper atmosphere.
(ii) Bleaching property : Ozone acts as a good bleaching agent due to its oxidising nature.
O3 → O + O2
Coloured matter + O → colourless matter
Ozone bleaches in absence of moisture so it is also known as dry bleach.
(iii) Reducing property : Ozone reduces peroxides to oxides. e.g.
H2O2 + O3 → H2O + 2O2
BaO2 + O3 → BaO + 2O2
(iv) Ozone depletion : Thinning of ozone layer in upper atmosphere is called ozone depletion.
- The ozone (O3) layer in the upper atmosphere, absorbs harmful UV radiations from the sun, thus protecting people on the earth.
- Depletion of ozone layer in the upper atmosphere is caused by nitrogen oxide released from exhausts system of car or supersonic jet aeroplanes.
NO (g) + O3 (g) → NO2 (g) + O2 (g)
- Depletion (thining) of ozone layer can also be caused by chlorofluoro carbons (freons) used in aerosol and refrigerators and their subsequent escape into the atmosphere.
- The depletion of ozone layer has been most pronounced in polar regions, especially over Antarctica.
- Ozone depletion is a major environmental problem because it increases the amount of ultraviolet (UV) radiation that reaches earth’s surface, thus causing an increase in rate of skin cancer, eye cataracts and genetic as well as immune system damage among people.
Q. How do the coolents in refrigerants deplete the concentration of ozone?
- The coolents used in refrigerants are generally chlorofiuorocarbons, CFCs also known as Freon, for example CF2Cl2.
- CFCs have very long lifetime about 20 to 100 years.
- CF2Cl2 undergoes photochemical decomposition giving free radicals which react and destroy ozone.
CF2Cl2 \(\underrightarrow{hv}\) CF2Cl. + Cl.
Cl. + O3 → ClO. + O2
ClO. + O3 → Cl. + 2O2
- Thus Cl. propagates the destruction of ozone in the atmosphere.
| Know This :
Ozone reacts with unsaturated compounds containing double bonds to form addition products called ozonides. Ozonides are decomposed by water or dilute acids to give aldehydes or ketones. This reaction is termed as ozonolysis |
Compounds of sulfur :
Sulfur dioxide :
Sulphur dioxide, SO2 is prepared by following methods :
(i) From sulfur : Sulfur dioxide gas can be prepared by burning of sulfur in air.
S(s) + O2(g) → SO2(g)
(ii) From sulfite : In the laboratory sulfur dioxide is prepared by treating sodium sulfite with dilute sulfuric acid.
Na2SO3 + H2SO4(aq) → Na2SO4+H2O(l)+ SO2(g)
(iii) From sulfides : (Industrial method) :
Sulfur dioxide can be prepared by roasting zinc sulfide and iron pyrites.
2ZnS(s) + 3O2(g) \(\underrightarrow{Δ}\) 2ZnO(s) + 2SO2(s)
4FeS2(s) + 11O2 (g) \(\underrightarrow{Δ}\) 2Fe2O3(s) + 8SO2(g)
Physical properties of SO2 :
- Sulfur dioxide is a colourless gas with a pungent smell.
- It is poisonous in nature.
- SO2 is highly soluble in water and its solution in water is called sulfurous acid.
- It liquifies at room temperature under a pressure of 2 atm and boils at 263 K.
Chemical Properties :
(i) Reaction with Cl2 : Sulfur dioxide reacts with chlorine in the presence of charcoal (catalyst) to form sulfuryl chloride.
SO2 (g) + Cl2 (g) \(\underrightarrow{Charcaol}\) SO2Cl2 (l)
(ii) Reaction with O2 : Sulfur dioxide is oxidised by dioxygen in presence of vanadium (V) oxide to sulfur trioxide.
2SO2 (g) + O2 (g) \(\underrightarrow{V_2O_5}\) 2SO3(g)
(iii) Reaction with NaOH : Sulfur dioxide readily reacts with sodium hydroxide solution to form sodium sulfite.
2NaOH + SO2 → Na2SO3 + H2O
(iv) Reaction with Na2SO3 : When SO2 gas is passed through sodium hydroxide solution (NaOH), it forms sodium sulphite, Na2SO3 which further with excess of SO2 forms sodium hydrogen sulphite, NaHSO3.
Na2SO3 + H2O(l) + SO2 → 2NaHSO3
(v) Reducing property :
- Sulfur dioxide acts as a reducing agent in the presence of moisture.
- Moist sulfur dioxide reduces ferric salts into ferrous salts.
2Fe3+ + SO2 + 2H2O → 2Fe2+ + SO42− + 4H+
- Moist sulfur dioxide decolourises acidified potassium permangnate (VII) solution.
2KMnO4 + 5SO2 + 2H2O → K2SO4 + 2MnSO4 + 2H2SO4
- Moist sulfur dioxide reduces halogens to halogen acids.
I2 + SO2 + 2H2O → H2SO4 + 2HI
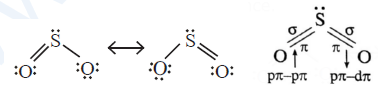
Structure of sulphur dioxide :
- SO2 molecule has a bent V shaped structure with S—O—S bond angle 1l9.5° and bond dissociation enthalpy is 297 kJmol−1.
- Sulphur in SO2 is sp2 hybridised forming three hybrid orbitals. Due to lone pair electrons, bond angle is reduced from 120° to 119.5°.
- In SO2, each oxygen atom is bonded to sulphur by a σ and a π
- σ bonds between S and O are formed by Sp2—p overlapping.
- One of π bonds is formed by p π—pπ overlapping while other π bond is formed by pπ—dπ
- Due to resonance both the bonds are identical having observed bond length 143 pm due to resonance.
Uses of sulphur dioxide :
- SO2 is used in the manufacture of H2SO4.
- In refining petroleum and also in sugar industry.
- In the manufacture of NaHSO3.
- As an antichlor, as a disinfectant and preservative.
- Liquid SO2 is used as a selective solvent to dissolve many inorganic and organic compounds.
- Sulphur dioxide is used for bleaching wool and silk.
As a bleaching agent in moist condition due to reduction reaction (SO2 + 2H2O → H2SO4+2[H])
colouring matter + [H] → colourless matter.
On exposing the bleached matter, the colour is restored due to oxidation.
Colourless matter + [O] → coloured matter.
Hence the bleaching action is temporary.
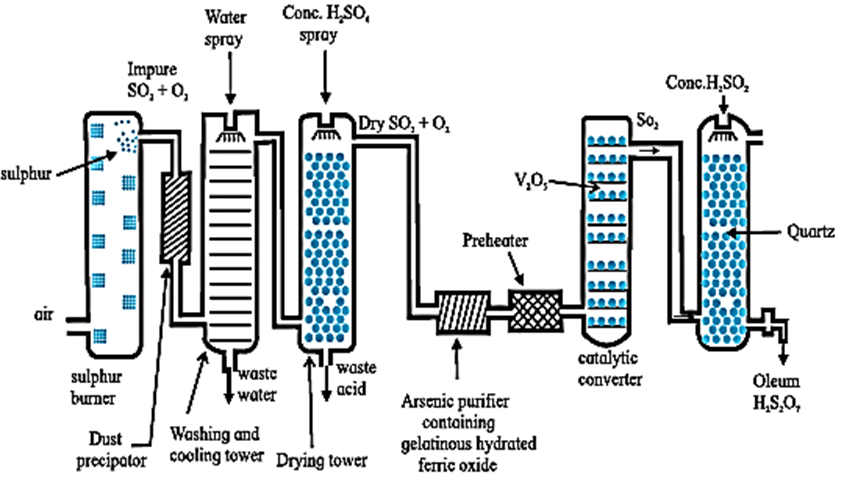
Sulfuric acid, H2SO4 :
Preparation : Sulfuric acid is manufactured by Contact process, which involves the following three steps.
(i) Preparation of SO2 : Sulfur or sulfide ore (iron pyrites) on burning or roasting in air produces sulfur dioxide.
S(s) + O2(g) \(\underrightarrow{Δ}\) SO2(g)
4FeS2(s) + 11O2 (g) \(\underrightarrow{Δ}\) 2Fe2O3(s) + 8SO2 (g)
(ii) Oxidation of SO2 to SO3 : Sulfur dioxide is oxidised catalytically with oxygen to sulfur trioxide, in the presence of V2O5 catalyst.
2SO2(g) + O2 \(\underrightarrow{V_2O_5}\) 2SO3(g)
The reaction is exothermic and reversible and the forward reaction leads to decrease in volume. Therefore low temperature (720K) and high pressure (2 bar) are favourable conditions for maximum yield of SO3.
(iii) Dissolution of SO3 : Sulfur trioxide gas (from the catalytic converter) is absorbed in concentrated H2SO4 to produce oleum.
Dilution of oleum with water gives sulfuric acid of desired concentration.
SO3(g) + H2SO4 → H2S2O7 (oleum)
H2S2O7 + H2O → 2H2SO4
The sulfuric acid obtained by contact process is 96 - 98 % pure.
Q. How is a solution of H2SO4 prepared from concentrated sulphuric acid solution?
Physical properties of H2SO4 :
- It is a colourless, dense, oily liquid having specific gravity 1.84 at 298 K.
- It has freezing point 283 K and boiling point 611 K.
- It is a strong dehydrating agent and dissolves in water with the evolution of a large amount of heat.
- It is a strong dibasic acid and acts as an oxidizing agent.
- Due to hydrogen bonding, it is viscous.
- It is highly corrosive and produces severe burns on skin.
Chemical Properties of H2SO4 :
(i) Acidic Property : Sulfuric acid ionises in aqueous solution in two steps.
H2SO4 (aq) + H2O(l) → H3O+(aq)+ HSO4(aq) ..(Ka > 10)
HSO4(aq)+ H2O(l) → H3O+ (aq) + SO42−(aq) …(Ka = 1.2 × 10−2)
The greater value of Ka (Ka>10) means that H2SO4 is largely dissociated into H+ and HSO4 ions. Thus H2SO4 is a strong acid.
(ii) Reaction with metals and nonmetals (oxidising property) : Metals and nonmetals both are oxidised by hot, concentrated sulfuric acid which itself gets reduced to SO2.
Cu + 2H2SO4 (Conc.) → CuSO4 + SO2 + 2H2O
S + 2H2SO4(Conc.) → 3SO2 + 2H2O
C + 2H2SO4(Conc.) → CO2 + 2SO2 + 2H2O
(iii) Dehydrating property : Concentrated sulfuric acid is a strong dehydrating agent.
Sulfuric acid removes water from sugar and carbohydrates. Carbon left behind is called sugar charcoal and the process is called charring.
C12H22O11 conc. → H2SO4 12C + 11H2O
(iv) Reaction with salts : Concentrated sulfuric acid decomposes the salts of more volatile acids to the corresponding acid
e.g.
NaCl + H2SO4 → NaHSO4 + HCl
KNO3 + H2SO4 → KHSO4 + HNO3
CaF2 + H2SO4 → CaSO4 + 2HF
Remember : Oxidizing properties of sulfuric acid depend on its concentration and temperature. In dilute solutions, at room temperature, H2SO4 behaves like HCl, oxidizing metals that stand above hydrogen in the e.m.f. series.
Fe(s) + 2H+(aq) → Fe2+(aq) + H2(g)
Hot, concentrated H2SO4 is a better oxidizing agent than the dilute, cold acid. It oxidises metals like copper.
Uses of H2SO4 :
Chapter-7-Elements of Groups 16, 17, and 18-Text Book-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Notes-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Solution-PDF
All 16 Chapters Notes -Class-12-Chemistry (16-PDF) Rs.137-Buy
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6-Chemical Kinetics – Online Notes
Next Chapter : Chapter-8-Transition and Inner transition Elements – Online Notes

We reply to valid query.