Adsorption and Colloids
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -11
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
Absorption : The phenomenon of uniform distribution of one substance throughout the body of another substance is called absorption. For example, absorption of ammonia by water.
Phenomenon of adsorption : The surfaces of every object around us are exposed to the atmosphere. Water molecules and other gas molecules from the air, such as N2, O2, form an invisible multi molecular layer on these items. This is known as the phenomenon of adsorption.
Examples of surface phenomenon :
- When a metal spoon dipped in milk is taken out, it is noticed that milk particles cover the spoon
- When a cold water bottle is removed from the refrigerator, water vapour condenses on the bottle's exterior surface.
- After the use, the clothes become dirty due to sticking of dust particles on the surface of the clothes.
Difference in the properties of a surface molecule and the bulk or a phase molecule.
| Surface molecule | Bulk molecule |
| At the surface the molecules from two different phases are in contact with each other. | Inside the bulk every molecule is surrounded or in contact with other molecules in the same phase. |
| A particle on the surface of a solid or a liquid is not surrounded symmetrically by other particles. | A particle (atom or a molecule) present in the bulk of a solid or a liquid is surrounded by other particles from all sides. |
| The surface particles are attracted partially and hence have unbalanced upper side attractive forces. | Bulk particles are uniformly attracted from all the sides and have their forces satisfied |

Hence the surface particles exert attractive forces on the particles of the other species in contact and results in the accumulation of the particles on the surface which gives rise to the phenomenon of adsorption.
Adsorption :
Unblanced forces :
The molecular forces at the surface of a liquid are unbalanced or in unsaturation state. In solids, the ions or molecules at the surface of a crystal do not have their forces satisfied by the close contact with other particles.
Adsorption : The accumulation of a substance on the surface of another substance due to unsatisfied or unbalanced attractive forces on the surface is called adsorption.
- The adsorption phenomenon is caused by unbalanced forces on the surface of a bulk involving London-dispersion forces or van der Waals forces. These are short range forces.
- There is a tendency for a liquid to decrease the surface tension and free energy and this leads to adsorption phenomenon.
Examples of adsorption.
- Adsorption of gases like hydrogen, oxygen, by finely divided metals, namely, platinum, palladium, copper, nickel, etc.
- Adsorption of gases like nitrogen, carbondioxide, by activated charcoal.
- Adsorption of acetic acid on activated charcoal.
- Removal of colouring dye like methylene blue by charcoal.
Comparison between Adsorption and Absorption :
| Adsorption | Absorption |
| 1. Adsorbed matter is concentrated only at the surface and does not penetrate through the surface to the bulk of adsorbent. Adsorption is a surface phenomenon. | Absorbed matter is uniformly distributed inside as well as on the surface of the bulk of substance. Absorption is a bulk phenomenon. |
| 2. Concentration of the adsorbate is high only at the surface of the adsorbent. | Concentration of the absorbate is uniform throughout the bulk of the absorbent. |
| 3. It is dependent on temperature and pressure. | It is independent of temperature and pressure. |
| 4. It is accompanied by evolution of heat known as heat of adsorption. | It is not accompanied by evolution or absorption of heat. |
| 5. It depends on surface area | It is independent of surface area. |
| 6. Example: Adsorption of a gas or liquid like acetic acid by activated charcoal | Example : Absorption of water by cotton. Absorption of ink by blotting paper. |
Terms involved in adsorption :
- Adsorbent : The material or substance present in the bulk, on the surface of which adsorption takes place is called adsorbent.
- Adsorbate : The substance getting absorbed on the the adsorbent is called as adsorbate.
Desorption: The process of removal of an adsorbed substance from a surface on
which it was adsorbed is called desorption.
Sorption: When, both adsorption and absorption occur simultaneously it is known as sorption.
- When a chalk is dipped in ink, ink molecules are adsorbed at the surface of chalk and the surface becomes coloured, while the solvent of the ink goes deeper into the chalk due to absorption.
Types of adsorption:
There are mainly two types of adsorption phenomenon depending on nature of forces involved.
- (i) Physical Adsorption or physisorption
- (ii) Chemical Adsorption or Chemisorption
(i) Physical Adsorption or physisorption :
- When the adsorbate such as gas molecules are accumulated on the surface of a solid due to weak van der Waals forces, the adsorption is called physical adsorption or physisorption.
- The heat released during physisorption is of the same order as that of condensation.
- The physisorption is weak in nature.
- The adsorbed gas forms several layers of molecules at high pressures.
- The extent of adsorption is large at low temperature.
- Physical adsorption is reversible.
(ii) Chemical Adsorption or Chemisorption :
- When the adsorbate like gas molecule are accumulated on the surface of a solid or on an adsorbent due to chemical bonds (covalent or ionic) then adsorption is called chemical adsorption or chemisorption.
- Chemisorption is specific in nature.
- It has high heat of adsorption.
- It involves a large energy of activation hence called activated adsorption.
- Chemisorption increases with the increase in temperature and after reaching maximum further increase in temperature decreases the extent of adsorption.
- Chemisorption forms monomolecular layer of adsorbed particles.
Comparison of physisorption and chemisorption :
| Physisorption | Chemisorption |
| 1. The forces operating are weak van der Waals forces. | 1. The forces operating are chemical nature (covalent or ionic bonds). |
| 2. Not specific in nature. All gases adsorb on all solids. For example, all gases adsorb on charcoal. | 2. Highly specific and occurs only when chemical bond formation is possible between adsorbent and adsorbate. For example, adsorption of oxygen on tungsten, hydrogen on nickel, etc. |
| 3. The heat of adsorption is low and lies in the range 20 - 40 kJ mol-1 | 3. Higher heat of adsorption and lies in the range 40 - 200 kJ mol-1 |
| 4. Occurs at low temperature and decreases with an increase of temperature. | 4. Favoured at high temperature, the extent of chemical adsorption is lowered at very high temperature, due to bond breaking. |
| 5. For example : at low temperature N2 gas is physically adsorbed on iron. | 5. For example N2 gas chemically adsorbed on iron at high temperature forms a layer of iron nitride, which desorbs at very high temperature. |
| 6. Reversible. | 6. Irreversible. |
| 7. Physisorbed layer may be multimolecular layer, of adsorbed particles under high pressure. | 7. Chemisorption forms monomolecular layer of adsorbed particles. |
Factors affecting adsorption of gases on solids :
(i) Nature of adsorbate (gas) :
- The amount of gas adsorbed by a solid depends on the nature of the gas.
- Gases having high critical temperature liquify easily and can readily be
- Gases that are easily liquefiable, such as SO2, Cl2, and NH3, are adsorbed to a greater extent than gases that are difficult to liquefy, such as N2, O2, and H2.
(ii) Nature of an adsorbent :
- Since the adsorption is a surface phenomenon, the substances which provide large surface area for a given mass are better adsorbents and would adsorb to a greater
- For example, Silica gel, activated charcoal are more effective adsorbents.
(iii) Surface area of an adsorbent :
- Adsorption is a surface phenomenon, hence as the surface area increases, the extent of adsorption increases.
- For example, finely divided substances like charcoal powder, rough metal surfaces, colloidal substances, etc. are good adsorbents.
(iv) Temperature :
- Adsorption is an exothermic process, hence according to Le Chatelier’s principle, it is favoured at low temperature.
- The amount of the gas adsorbed decreases as the temperature increases.
- The Fig. shows that the volume of N2 gas adsorbed per unit mass of an adsorbent at constant pressure decreases as the temperature increases.
(v) Pressure of a gas :
- The extent of adsorption of a gas on a solid adsorbent at constant temperature increases with the increase in pressure. This is because at higher pressure more gas molecules (adsorbate) come in close contact with adsorbent particles (atoms or molecules).
- However, at high pressure, the surface area of the adsorbent is almost fully covered by the adsorbed gaseous molecules.
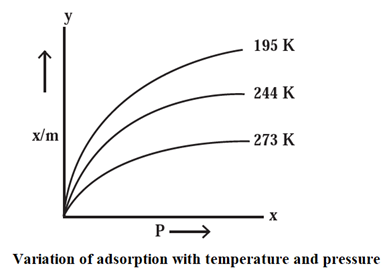
Adsorption Isotherm :
Adsorption Isotherm : The relationship between the amount of a substance adsorbed per unit mass of an adsorbent and the equilibrium pressure (in case of gas) or concentration (in case of solution) at pa given constant temperature is called an adsorption isotherm.
Freundlich adsorption isotherm :
The empirical equation representing the relationship between the amount of the substance (adsorbate) adsorbed per unit mass of an adsorbent and the equilibrium pressure at constant temperature is called Freundlich adsorption isotherm.
Mathematical Expression :
Freundlich proposed an empirical equation for adsorption of a gas on solid.
x/m = kP1/n (n>1)
Where x = mass of the gas adsorbed
m = mass of the adsorbent at presure P
x/m = mass of gas adsorbed per unit mass of adsorbent
P = equilibrium pressure.
k and n are constants which depend on the nature of adsorbate, adsorbent and temperature.
In case of solution, P in the equation is replaced by the concentration.
∴ x/m = kC1/n , where C is equilibrium concentration
Graphical Representation of Freundlich equation :
(1) When x/m is plotted against P, a smooth parabolic curve is obtained.
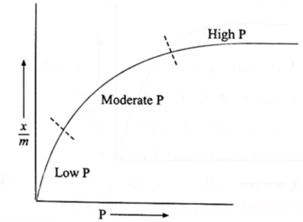
(i) At low pressure : x/m increases linearly with the increase in pressure,
Hence x/m ∝ P1.
(ii) At high pressure : Adsorption is independent of P.
Hence x/m ∝ P0.
(m) At moderate pressure : x/m varies exponentially.
x/m ∝ P1/n where 0 < 1/n < 1.
(2) Taking the logarithm to the base 10 of both sides of Freundlich’s isotherm (equation),
log10 \(\frac{x}{m}\) = \(\frac{1}{n}\) log10 C + log10 k
This is an equation of a straight line (y = mx + c).
If log10 \(\frac{x}{m}\) is plotted against log10 P, a straight line is obtained.
The slope of this line is \(\frac{1}{n}\) and the intercept on log10 \(\frac{x}{m}\) axis is log10 k

For a solution we can write,
log10 \(\frac{x}{m}\) = \(\frac{1}{n}\)log10 C + log10 k
Applications of Adsorption :
The adsorption finds large number of applications as illustrated here.
(i) Catalysis:
The solid catalysts are used in many industrial manufacturing processes.
Examples :
- Iron is used as the catalyst in manufacturing of ammonia;
- Platinum is used in manufacturing of sulphuric acid, H2SO4 (by contanct process).
- In hydrogenation of oils, finely divided nickel is employed as catalyst.
(ii) Gas masks: It is a device which consists of activated charcoal or mixture of adsorbents. It is used for breathing in coal mines to avoid inhaling of the poisonous gases.
(iii) Control of humidity : Silica and alumina gels are good adsorbents of moisture.
(iv) Production of high vacuum : Lowering of temperature at a given pressure,
increases the rate of adsorption of gases on charcoal powder. By using this principle, high vacuum can be attained by adsorption.
- A vessel evacuated by vacuum pump is connected to another vessel containing coconut charcoal cooled by liquid air. The charcoal adsorbs the remaining traces of air or moisture to create a high vacuum.
(v) Adsorption Indicators : The adsorption is used to detect the end point of precipitation titrations. Dyes such as eosin, fluorescein are used as indicators.
For example, A solution of sodium chloride containing a small amount of fluorescein is titrated against silver nitrate solution.
NaCl + AgNO3 → AgCl + NaNO3
White ppt
When chloride ions are over, fluorescein is adsorbed on white silver chloride precipitate and red colour is developed. Thus colour change from pale yellow to reddish pink is observed at the end point.
(vi) Separation of inert gases : In a mixture of noble gases, different gases adsorb to different extent. Due to selective adsorption principle, gases can be separated on coconut charcoal.
(vii) Froth floatation process : A low grade sulfide ore is concentrated by separating it from silica and other earthy matter using pine oil as frothing agent. Hydrophobic pine oil preferentially wets (adsorbs on) sulfide ore which is taken up in the froth.
(viii) Chromatographic analysis : It is based on selective adsorption of ions from
solution using powdered adsorbents such as silica or alumina gel.
- It has several industrial and analytical applications.
- Other applications: Surface area determination, purification of water, etc.
Catalysis :
Catalyst : A substance which, when added to the reacting system increases the rate of a reaction without itself undergoing any permanent chemical change.
Catalysis : The phenomenon of increasing the rate of a chemical reaction with the help of a catalyst is known as catalysis.
There are two types of catalysis :
- Homogeneous catalysis ;
- Heterogeneous catalysis.
Homogeneous Catalysis : When the reactants and the catalyst are in the same phase, it is said to be homogenous catalysis.
Examples of homogeneous catalysis:
(i) Iodide ion (I—) finds use as homogeneous catalyst in decomposition of aqueous hydrogen peroxide
(ii) Oxidation of sulfur dioxide to sulfur trioxide with dioxygen (O2) in the presence of nitirc oxide as catalyst (lead chamber process).
2SO2 (g) + O2 (g) \(\underrightarrow{NO(g)}\) 2SO3 (g)
(iii) Hydrolysis of sugar is catalysed by H+ ion furnished by sulphuric acid.
C12H22O11 (aq) + H2O (l) \(\underrightarrow{H_2SO_4}\) C6H12O6 (aq) + C6H12O6 (aq)
Sucrose solution → Glucose + Fructose
(iv) Enzyme catalysis is also an example of important type of homogeneous catalysis.
Heterogeneous catalysis : When the reactant and catalyst are in different phase, it is said to be heterogeneous catalysis.
- In this case, the catalysts exist in difference phase from that of the reactants.
- The heterogeneous catalyst is generally a solid and the reactants are gases or liquids.
- In this, the reactants are adsorbed on the surface of the catalyst by forming chemical bonds.
- Finally when the products are formed, they are desorbed from the surface.
Examples of heterogeneous catalysis:
(i) Dinitrogen (N2) and dihydrogen (H2) combine to form ammonia in Haber process in presence of finely divided iron along with K2O and Al2O3.
N2 (g) + 3H2(g) \(\underrightarrow{Fe(s)K_2O,Al_2O_3}\) 2NH3 (g)
Here Al2O3 and K2O are promoters of the Fe catalyst. Al2O3 is added to prevent the fusion of Fe particles. K2O causes chemisorption of nitrogen atoms. Molybdynum is also used as promoter.
(ii) Hydrogenation reaction of vegetable oils to produce solid fat is used in food industry. The reaction is catalysed by finely divided metals like Ni, Pd or Pt. Vegetable oil contains one or more carbon carbon double bonds (C = C) in its structure. On hydrogenation a solid product (which contains only carbon carbon single bonds) is formed. It is called vanaspati ghee.
Vegetable oil (l) + H2 (g) \(\underrightarrow{Ni(s)}\) Vegetable ghee(s)
(iii) Automobile catalytic converters are another prominent use for heterogeneous catalysts. A considerable number of air contaminants, such as carbon monoxide and nitric oxide, are found in automotive exhaust. The catalytic converter converts contaminants in the air into carbon dioxide, water, nitrogen, and oxygen. The adsorption of Pb (lead) poisons the catalyst. Catalytic converter-equipped vehicles require unleaded fuel.
Inhibition : The phenomenon of retarding a rate of a reaction in the presence of an inhibitor is called inhibition.
Inhibitors : Inhibitors are substances which decrease the rate of chemical reaction. Example:
(i) Use of 2% ethanol to prevent oxidation of chloroform.
- Chloroform was used as an anaesthetic. On oxidation, chloroform forms poisonous carbonyl chloride, COCl2.
4CHCl3(l)+3O2(g) → 4COCl2(g) + 2H2O + 2Cl2(g)
- On addition of 2% ethanol, the formation of poisonous COCl2 is suppressed. In this, ethanol acts as an inhibitor.
(ii) H2O2 (l) decomposes as,
2H2O2(l) → 2H2O(l) + O2(g)
- This decomposition can be inhibited by addition of dilute acid or glycerol which act as inhibitors.
Adsorption Theory of Heterogeneous catalysis:
The catalytic action occurs on the surface of a catalyst. The mechanism involves
five steps.
- Diffusion of reactants toward the surface of the catalyst.
- Adsorption of reactant molecules on the surface of the catalyst.
- Occurrence of chemical reaction on the catalyst surface and formation of an intermediate.
- Formation of products.
- Desorption of reaction products from the catalyst surface. Products leave catalyst surface.
The steps involved can be shown as :
Diffusion → Adsorption → intermediate formation → Product formation → Desorption Fresh reactant molecules can replace the products to start the cycle again as in step (i). This explains why catalyst remains unchanged in mass and chemical composition at the end of the reaction.
Important features of solid catalysts:
(1) Catalytic activity: The catalytic activity of a solid catalyst arises due to chemical adsorption of reactant molecules on the surface of the catalyst. Hence this activity depends upon the strength of adsorption. An active catalyst adsorbs the reactants to a greater extent.
Strongly active catalysts are d-block metals like Fe, V, Cr, etc. which adsorb O2, C2H2, C2H4, H2, N2, etc. Mn and Cu do not adsorb N2 and CO2. Mg and Li adsorb O2 selectively.
(2) Finely divided substance as adsorbent : These small size particles provide a large surface area compared to a large particle. Hence, adsorption is to a greater extent. This increases the catalytic activity to a greater extent.
(3) Catalytic selectivity : Many catalysts are selective in their action. The formation of products depends upon the nature of the catalyst.
For example,
(i) The gaseous ethylene and O2 react to produce different products with different catalysts.
(a) C2H4(g) + O2(g) \(\underrightarrow{Pd/Al_2O_3}\) 2CH3CHO(g)
(ethylene) (acetaldehyde)
(b) 2C2H4(g) + O2(g) \(\underrightarrow{Ag/Al_2O_3}\) 2C2H4O(g) (ethylene oxide)
(ii) The gaseous carbon monoxide and H2 produce different products by using different catalysts.
(a) CO(g) + 3H2(g) \(\underrightarrow{Ni}\) CH4(g) + H2O(g)
(b) CO(g) + 2H2(g) \(\underrightarrow{Cu/ZnO.Cr_2O_3}\) CH3OH(g)
(4) Selective catalysis by zeolites : Zeolites are alumino silicates with three-dimensional network of silicates. Some silicon atoms in this network are replaced by aluminium atoms giving Al-O-Si frame work. This results in microporous structure. The reactions in zeolites are dependent on the size and shape of reactant or products and also on pores and cavities of zeolites. Zeolites, therefore, are shape selective catalysts.
In petroleum industry, zeolite catalyst ZSM-S converts alcohols directly to gasoline (petrol) by dehydration which gives a mixture of hydrocarbons.
Class-11-Chemistry-Chapter-11-Adsorption and Colloids-Text Book
Class-11-Chemistry-Chapter-11-Adsorption and Colloids- Notes
Class-11-Chemistry-Chapter-11-Adsorption and Colloids-Solution
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-10-States of Matter – Online Notes
Next Chapter : Chapter-12-Chemical Equilibrium – Online Notes