Some Basic Concepts of Chemistry
Class-11-Science-Chemistry-Chapter-1-Maharashtra State Board
Notes
|
Topics to be Learn :
|
Chemistry : Chemistry is the study of physical and chemical properties of matter and the physical and chemical changes it undergoes under different conditions.
Chemistry is classified into five different branches as follows :
- Organic chemistry: This is the study of the properties and reactions of compounds of carbon.
- Inorganic chemistry: This is the study of all substances which are not organic.
- Physical chemistry : This is the study of principles underlying chemistry. It involves the studies of properties of matter atoms, molecules and fundamental concepts in chemistry.
- Biochemistry: This involves the study of the chemistry of living organisms, the structure and function of their chemical components.
- Analytical chemistry : The branch of chemistry which deals with the study of separation, identification, qualitative and quantitative determination of the compositions of different substances is called analytical chemistry.
Matter : Matter occupies space and has mass. It may exist in different states namely solid, liquid and gaseous.
Matter can be classified into two categories,
- Pure substances : Pure substances have a definite chemical composition. They always have the same properties regardless of their origin. They are divided into elements and compounds.
- Mixtures : Mixtures have no definite chemical compositions and hence no definite properties.
Elements : Elements are pure substances which can not be broken down into simpler substances by ordinary chemical changes. Elements are classified as Metals, Non-metals and Metalloids
Metals :
- They have a lustre.
- They conduct heat and electricity.
- They can be drawn into wire hence they are ductile.
- They can be hammered into thin sheets hence they are malleable.
- Example, gold, silver, etc.
Non-metals :
- They have no lustre.
- They are poor conductors of heat and electricity.
- They are not ductile and malleable.
- Example, iodine, carbon, etc.
Metalloids : These elements have intermediate properties between metals and non-metals. For example, As, Si, etc.
Compound: Compound is a pure substance which cannot be broken down into simpler substances by ordinary chemical changes. Each compound is formed from two or more elements combined in a fixed proportion.
Mixtures are classified as follows :
- Homogeneous mixture : It forms one phase uniformly mixed mixture throughout its bulk. For example sugar solution.
- Heterogeneous mixture : It involves a mixture of components in different phases which are not uniformly distributed throughout the bulk. For example, a mixture of water and oil.
Examples of Mixtures, Pure substances, Elements and Compounds
Mixtures
Pure substances
Elements
Compounds
Physical and Chemical properties of a matter :
- Physical properties : These are the properties which can be measured or observed without changing the chemical composition of the matter. For example, colour, odour, melting point, boiling point, density, etc.
- Chemical properties : These are the properties which undergo a change when the matter undergoes a chemical change. For example, Mg when changes to MgO by oxidation its properties change.
Units :T1le arbitrarily decided and universally accepted standards to measure physical quantity of a matter are called units.
The system of units is based upon three basic units namely length, mass and time.
There are three different systems of units as follows :
- Centimetre-Gram—Second (CGS) System : In this system, the unit of length is centimetre, the unit of mass is gram and the unit of time is second.
- Foot—Pound—Second (FPS) System : In this system, the units of length, mass and time are foot, pound and second respectively.
- Metre—Kilogram—Second (MKS) System : In this system, the units of length, mass and time are metre, kilogram and second respectively.
SI units : In 1960, the 11th General Conference of Weights and Measures adopted an International System of units, called SI units (French name Le Systme International d’Unit’es) which has seven fundamental units.
Fundamental units : These are independent units and not derived from any other units.
There are seven fundamental units namely
(i) length, (ii) mass, (m) time, (iv) thermodynamic temperature, (v) amount of substance, (vi) electric current and (vii) luminous intensity.
Fundamental SI units and symbols :
Fundamental quantity
SI units
symbols
1) Length
metre
m
2) Mass
kilogram
Kg
3) Time
second
s
4) Temperature
kelvin
K
5) Electric current
ampere
A
6) Luminous Intensity
candela
cd
7) Amount of substance
mole
mol
Derived units : The units of the physical quantities derived from seven fundamental units are known as derived units. For example, Area, volume, density, velocity, etc.
Derived quantities, Special names in SI of the units and symbol :
Physical quantity
SI Unit
Symbol, name of the scientist
Equivalent combination of other SI units
Force
newton, N
Isaac Newton
kg.m/s2
Energy, work
joule, J
James Joule
N-m=kg-m2/s2
Power
watt, W
James Watt
J/s= kg-m2/s2
Pressure
pascal, Pa
Blaise Pascal
N/m2=kg/s2.m
Frequency
hertz, Hz
Heinrich Hertz
s-1
Electric charge
couloumb, C
Charles Couloumb
A.s
Electric potential
volt, V
Alessandro Volt
J/A.s=kg.m2/s3.A
Electric resistance
ohm, W
George Ohm
V/A= kg.m2/s3.A2
Magnetic induction
tesla, T
Nikola Tesla
Wb/m2=kg/s2.A
Mass and weight:The mass is an inherent property of matter which is the measure of quantity of matter in the body. It does not vary with its position.
The weight of a body varies with position since it depends upon gravitational attraction of earth.
Therefore mass of a body is more fundamental property than its weight.
(1 kg = 1000 g)
Length : In chemistry, length term arises in properties such as the atomic radius, bond length, wavelength, etc.
(1 nm=10-9 m; 1 pm=10-12 m)
Volume : It is the amount of space occupied by the matter.
(1 L = 1 dm3 = 1000 mL = 1000 cm3 = 10-3 m3)
Density : It is mass per unit volume of a substance.
CGS units : g mL-1 or g cm-3
SI units : kg m-3
Temperature : It is a measure of the hotness or coldness of an object. It is expressed in three common scales namely, degree celsius (°C), degree Fahrenheit (°F) and Kelvin (K).
Relation between Celsius and Fahrenheit :
°F = \(\frac 95\) °C + 32
OR \(\frac{°C}{5}=\frac{°F-32}{9}\)
Law of conservation of mass : This law states that during chemical combination of matter, the mass is neither created nor destroyed.
Explanation : Antoine Lavoisier a French scientist while studying several combustion reactions, determined accurately the masses of the reactants before reactions and the masses of the products after the completion of the reactions. He observed that the total masses of the reactants before the reaction is equal to the total masses of the products. For example 1) When hydrogen gas burns and combines with oxygen to yield water, the mass of the water formed is equal to the mass of the hydrogen and oxygen consumed. Hydrogen + Oxygen —> Water 2 g 16 g 18 g 2) When solid carbon burns and combines with oxygen to form carbon dioxide, The total mass of carbon (12 g) and oxygen taken initially (32 g) is equal to the mass of Carbon dioxide (44 g) formed. C + O2 —> CO2 (s) (g) (g) (12g) (32g) —> (44g) Applications :
The law of definite composition (or proportion) : This law states that any pure compound always contains the same elements in a definite composition (or proportion) by weight irrespective of the source or method of its preparation.
Explanation : Irrespective of a source or a method of preparation, water (H2O) always contains 11.11 % hydrogen and 88.89 % oxygen, ammonia (NH3) always contains 17.64 % hydrogen and 82.36 % nitrogen by weight.
The law of multiple proportions : This law states that when two elements chemically combine to form two or more compounds with different compositions by weights, then the masses of one element that combine with a fixed mass of the other element are in the ratio of small whole numbers.
Explanation : Hydrogen combines with oxygen to form two compounds, namely water (H2O) and hydrogen peroxide (H2O2). Hydrogen + Oxygen ——> Water 2 g 16 g 18 g Hydrogen + Oxygen -—> Hydrogen Peroxide 2 g 32 g 34 g It is found that the two masses of oxygen namely 16 g and 32 g which combine with a fixed mass of hydrogen (2 g) are in the ratio of small hole numbers, i.e. 16 : 32 or 1 : 2.
Q.Show that the compounds, NO, NO2, N2O5 satisfy the law of multiple proportions.
Atomic masses of N and O are 14 and 16 respectively. We can calculate different masses of oxygen reacting with unit mass (1 gram) of nitrogen in different compounds. In NO, 14 gram nitrogen combines with 16 g oxygen, hence 1 gram nitrogen combines with, 16/14 = 1.143 gram of oxygen. In NO2, 14 gram nitrogen combines with 2 x 16 = 32 gram oxygen, hence 1 gram nitrogen combines with 32/14 = 2.286 gram oxygen. In N2O5, 2 x 14 =28 gram nitrogen combine with 5 x 16 = 80 gram oxygen, hence 1 gram nitrogen combines with 80/28 =2.857 gram oxygen. Therefore the ratio of masses of oxygen combining with a fixed mass of nitrogen i.e., 1 gram of nitrogen is, 1.143 : 2.286 : 2.86 i.e., 1 : 2 : 2.5. Multiplying by smallest integer 2, the ratio is, 2 : 4 : 5. Hence, the given compounds satisfy the law of multiple proportions.
Gay-Lussac’s Law of Gaseous Volume : This law states that when gases combine or are produced in a chemical reaction they do so in a simple ratio by volume, provided all gases are at same temperature and pressure.
Explanation : Under identical conditions of temperature and pressure 2 L of hydrogen gas combine with 1 L. of oxygen gas forming 2 L of water vapour.
2H2(g) + O2(g) --> 2H2O(g)
2 L 1 L 2 L
2 volumes : 1 volume --> 2 volumes
Hence, the volumes of H2(g) , O2(g) and H2O(g) are in the simple whole number ratio of 2 : 1 : 2.
Q. If 10 volumes of dihydrogen gas react with 5 volumes of dioxygen gas, how many volumes of water vapour would be produced ?
With reference to above explanation of Gay-Lussac’s Law of Gaseous Volume, 2 volumes of H2(g), combine with 1 volume of O2(g) and form 2 volumes of H2O(g), Similarly 10 volume of of H2(g), will combine with 5 volumes of O2(g) forming 10 volumes of H2O(g) ∴ 10 volumes of water vapour are produced.
Avogadro’s law : It states that equal volumes of different gases under identical conditions of pressure and temperature contain equal number of molecules.
Explanation: Consider the reaction between gaseous hydrogen and oxygen forming water vapour.
2H2(g) + O2(g) à 2H2O(g)
100 mL 50 mL 100 mL
2 volumes 1 volume 2 volumes
Since volume V of a gas is directly proportional to number of gas molecule (Vµn),
2 n molecules : n molecule : 2 n molecules OR
2 molecules : 1 molecule : 2 molecules
Thus 2 molecules of hydrogen combine with 1 molecule of oxygen to give 2 molecules of water vapour.
Dalton’s atomic theory : Dalton's theory could explain all the laws of chemical combination.
The assumptions of Dalton’s atomic theory are as follows :
- All matter is made up of tiny indivisible particles called atoms.
- All atoms of the same element have same size, shape and same properties, while atoms of different elements have different properties.
- Compounds are formed when atoms of different elements combine in a fixed ratio.
- Chemical reactions involve only the reorganization of atoms. Atoms are neither created nor destroyed in a chemical reaction.
Constituents of an atom and their masses : An atom consists of three fundamental particles namely, (1) electron, (2) proton and (3) neutron. In an atom, the protons and the neutrons are present in the nucleus of an atom while electrons revolve around the nucleus.
Magnitude of mass of an atom : The mass of an atom is due to the masses of three fundamental particles namely electrons, protons and neutrons present in the atom.
- Since these particles have extremely small masses, the mass of an atom is also very small, of the order of 10-24g or 10-27kg.
- The mass of one atom is also called atomic mass.
One amu : One amu is defined as a mass exactly equal to one twelth of the mass of one carbon-12 atom.
1 amu = \(\frac{1}{12}\)× mass of one C-12
= \(\frac{1}{12}\)×1.992648 x 10-23 g
= 1.66054 x 10-24 g
= 1.66054 x 10-27 kg
Recently amu has been replaced by unified mass unit (u) also called dalton (Da).
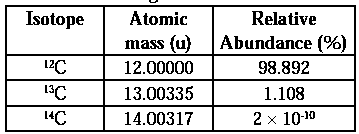
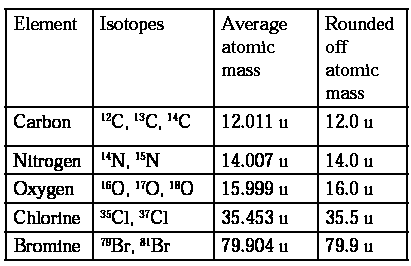
Average atomic mass : An element may have different isotopes i.e., atoms having same number of electrons and protons but different number of neutrons. The isotopes have different atomic masses and they exist in different proportion or abundance. The atomic mass of an element is the weighted average of atomic masses of its isotopes. This is called average atomic mass, Isotopes with relative abundances and atomic masses as shown against each of them. From the above data, the average atomic mass of carbon = (12 u) (98.892/100) + (13.00335 u) (1.108/100) + (14.00317) (2 × 10-10/100) = 12.011 u Similarly, average atomic masses for other elements can be calculated.
Remember:
|
Molecular mass : Molecular mass is the sum of average atomic masses of the atoms of an element which constitute the molecule.
The molecular mass is the mass of one molecule of that substance relative to the mass of one atom of carbon-12.
For example, the molecular mass of carbon dioxide (CO2) is
= 1(average atomic mass of C) + 2 (average atomic mass of O)
= 1 (12.0 u) + 2 (16.0 u) = 44.0 u
Some more examples of calculations of molecular mass.
i. H2O = 2 × 1 u + 16 u = 18 u
ii. C6H5Cl = (6 × 12 u) + (5 × 1 u) + (35.5 u) = 112.5 u
iii. H2SO4 =(2 × 1 u) + (32 u) +(4 × 16 u) = 98 u
Formula mass : It is the sum of atomic masses of the atoms present in the formula of the substance. Explanation : Consider the formula mass of NaNO3. Formula mass of NaNO3 = average atomic mass of Na + average atomic mass of N + 3 x average atomic mass of O =23 u + 14 u + 3 x 16u = 85u
Mole concept :
Mole : One mole of a substance is defined as the amount of a substance that contains the number of particles like atoms, molecules, ions or electrons equal to the number of carbon atoms present in 0.012 kg of Carbon-12 i.e., 6.0221367 x 1023 particles.
One mole of a substance contains 6.022 x 1023 molecules while one mole (or one gram atom) of an element contains 6.0221367 x 1023 atoms.
[Note : The name of the unit is mole and the symbol for the unit is mol.]
Remember :
|
Molar Mass :The mass of one mole of a substance (element / compound) in grams is called its molar mass. The molar mass of any element in grams is numerically equal to atomic mass of that element in u. Similarly molar mass of any substance, existing as polyatomic molecule, in grams is numerically equal to its molecular mass or formula mass in u.
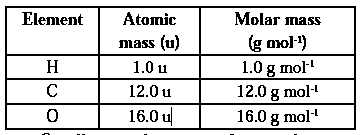

Q. Calculate the molar mass of oxygen atoms
Atomic mass of O = 16 u Molar mass of O atoms = NA x atomic mass of O = 6.022 x 1023 x 16 u = 6.022 x 1023 x 16 x 1.66054 x 10-24 g = 16.0 g mol-1 Molar mass of oxygen = 16.0 g mol-1.
Moles and gases :
STP condition :
STP represents standard temperature and pressure condition where, temperature is 0°C and pressure is 1 atm.
Molar volume of a gas : The volume of one mole of a gas is called molar volume. At STP condition (0°C and 1 atm), the molar volume of any gas is 22.4 dm3.
Volume of one mole of a gas at STP :
One mole of any gas occupies a volume of 22.4 dm3 at standard temperature and pressure condition.
Number of moles of a gas (n) = \(\frac{\text{Volume of the gas at STP}}{\text{Molar volume of gas}}\)
= \(\frac{\text{Volume of the gas at STP}}{22.4}\)
∴ Number of molecules = number of moles x NA
= number of moles x 6.022 x 1023 mol-1
[Note : If pressure is expressed in bar unit, i.e. P = 1 bar then the molar volume of the gas at STP is 22.71 L mol-1]
Q. Calculate the volume in dm3 occupied by 60.0 g of ethane at STP.
Molar mass of ethane, C2H6 = 2 x 12 + 1 x 6 = 30 g mol-1 Number of moles of C2H6 = W/M = 60/30 = 2 mol Because 1 mol of C2H6 at STP occupies 22.4 dm3 2 mol of C2H6 at STP will occupy, V = 2 x 22.4 = 44.8 dm3 Answer is, Volume of C2H6 = 44.8 dm3.
Important Terms, Units and Laws : Molecular mass (Molar mass) : The ratio of mass of one molecule of a substance to \(\frac{1}{12}\)th mass of one atom of Carbon-12. Avogadro number (NA) = 6.022 x 1023 mol 1 Mole of an element OR 1 Gram atom of an element contains 6.022 x 1023 atoms of the element. 1 Mole of a substance contains 6.022 x 1023 molecules of the substance. STP OR NTP condition : Pressure = 1 atmosphere = 1.013 x 105 Nm-2; Temperature = 273.15K Volume of 1 mole of a gas at STP = 22.414 L = 0.022414 m3 (A) Molecular formula weight =11 x Empirical formula weight (B) Molecular formula = n x Empirical formula.
Importance and scope of chemistry
Systems of units
Seven fundamental SI units
Laws of chemical combination
Atomic mass : The ratio of mass of one atom of an element to \(\frac{1}{12}\) th of mass of one atom of Carbon -12.
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Next Chapter : Chapter-2-Introduction to Analytical Chemistry – Online Notes
We reply to valid query.