Amines
Maharashtra Board-Class-12-Chemistry-Chapter-13
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
Amines : The alkyl or aryl derivatives of ammonia in which one, two or all the three hydrogen atoms attached to nitrogen are replaced by same or different alkyl or aryl groups are called amines. OR Amines are nitrogen containing organic compounds having basic character.
- Amines are present in structure of many natural compounds like proteins, vitamins, hormones and many plant products like nicotine.
Example : methyl amine : CH3-NH2
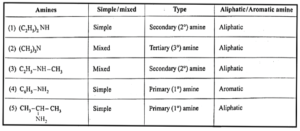
Classification of amines :
- Amines are classified on the basis of the number of hydrogen atoms of ammonia that are replaced by alkyl group.
- Amines are classified as primary (1°), secondary (2°) and tertiary (3°).

Primary amines (1° amines) : The amines in which only one hydrogen atom of ammonia is replaced by an alkyl group or aryl group are called primary (1°) amines.
Examples :

Secondary amines (2° amines) : The amines in which two hydrogen atoms of ammonia are replaced by two, same or different alkyl or aryl groups are called secondary (2°) amines.
Examples :
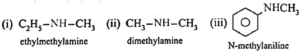
Tertiary amines (3° amines) : The amines in which all the three hydrogen atoms of ammonia are replaced by three same or different alkyl or aryl groups are called tertiary (3°) amines.
Examples :
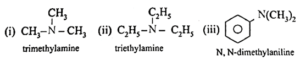
Secondary and tertiary amines are further classified as (i) simple / symmetrical amines and (ii) mixed / unsymmetrical amines.
(i) Simple or symmetrical amines : When all the alkyl or aryl groups on nitrogen are same, it is a simple amine.
Examples :
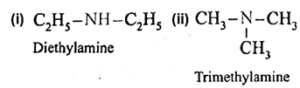
(ii) Mixed or unsymmetrical amines : When all the alkyl or aryl groups on nitrogen are different, then the amine is a mixed amine.
Examples :
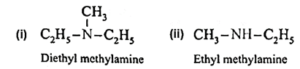
Amines are also divided into two major classes, namely, aliphatic and aromatic amines on the basis of nature of the groups attached to the nitrogen atom.
Functional group in : (1) Primary amine (2) Secondary amine (3) Tertiary amine

Nomenclature of Amines :
(A) Common Names : Rules
(i) According to common naming system, the amines are named as alkylamines.
(ii) The common name of a primary amine is obtained by writing the name of the alkyl group followed by the word ‘amine’.
Example : methyl amine : CH3-NH2
(iii) The simple (symmetrical ) secondary and tertiary amines are written by adding prefix ‘di’- (for presence of two alkyl groups) and ‘tri’- (for presence of three alkyl groups ) respectively to the name of alkyl groups.
Examples : CH3-NH-CH3 dimethylamine, (C2H5)3N triethylamine

(iv) The mixed (or unsymmetrical) secondary and tertiary amines are given names by writing the names of alkyl groups in alphabetical order, followed by the word ‘amine’.
Example : CH3—CH2—NH—CH3 ethylmethylamine
(B) IUPAC names : Rules
(i) According to IUPAC system of nomenclature of amines, aliphatic amines are named as alkanamines.
(ii) The name of the amine is obtained by replacing the suffix ‘e’ from parent alkane’s name by ‘amine’.
(iii) The position of the amino group is indicated by the lowest possible locant.
Examples :
(iv) In case of secondary and tertiary amines, the largest alkyl group is considered to be the parent alkane and other alkyl groups are written as N-substituents.
Example : C2H5NH-CH3 N—Methylethanamine
(v) In case of arylamines ending ‘e’ of arene is replaced by amine.
(vi) A complete name of amine is written as one word.
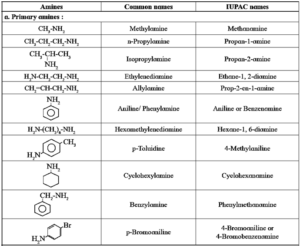
Common and IUPAC names of some alkyl and arylamines :


Preparation of Amines :
(i) By ammonolysis of alkyl halides :
- When alkyl halide is heated with alcoholic solution of excess ammonia it undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino (-NH2) group to form primary amine.
- This process of breaking of C-X bond by ammonia is known as ammonolysis. The reaction is also known as alkylation of ammonia.
- The reaction is carried out in a sealed tube at 373 K.
- In this reaction a mixture of primary, secondary, tertiary amines and a quaternary ammonium salt is obtained.
- It may be noted that the primary amine obtained in the 1st step is stronger nucleophile than ammonia.
- Hence, it further reacts with alkyl halide to form secondary and tertiary amines and finally quaternary ammonium salt if NH3 is not used in large excess.
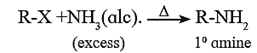
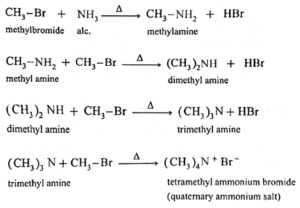
Example :
When methyl bromide is heated with alcoholic ammonia at 373 K, it gives a mixture of methylamine (a primary amine), dimethylamine (a secondary amine), trimethyl amine (a tertiary amine) and tetramethylammonium bromide (a quaternary ammonium salt).
- The order of reactivity of alkyl halides with ammonia is R-I > R-Br > R-Cl.
- In the laboratory, ammonolysis of alkyl halides is not a suitable method to prepare primary amines as it gives a mixture of primary, secondary, tertiary amines and quaternary ammonium salts. The separation of primary amine becomes difficult.
(ii) Reduction of nitrocompounds :
Aliphatic and aromatic nitrocompounds can be reduced to primary amines by using metal-acid mixture (Sn/HCl or Fe/HCl or Zn/HCl) or catalytic hydrogenation (H2/Ni or Pt or Pd) or LiAlH4 in ether.
When a nitroalkane is refluxed with tin (or iron) and concentrated HCl it gives corresponding primary amine.

Examples :
(a) nitromethane on reduction by refluxing with Sn and concentrated HCI gives methylamine.

(b) Nitrobenzene on reduction with tin and concentrated HCI or by using H,/Pd in ethanol gives aniline.
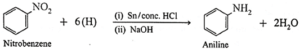
(c) When nitropropane is reduced in the presence of LiAlH, in ether, n-propyl amine is obtained.
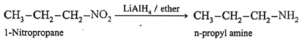
(iii) Reduction of alkyl cyanide (alkanenitriles) :
Alkyl cyanides (nitriles) on reduction by sodium and ethyl alcohol form corresponding primary amines. This reaction is called Mendius reduction.
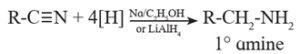
Examples :
(a) Propionitrile on reduction by sodium and ethanol gives n-propyl amine (Propan-1-amine).

(b) Methyl cyanide or acetonitrile on reduction by sodium and ethanol gives ethanamine.

(iv) By reduction of amides :
Acid amides on reduction with lithium aluminium hydride or sodium, ethanol form corresponding primary amines.
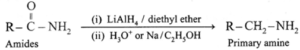
Examples :
(a) Acetamide on reduction with lithium aluminium hydride or sodium, ethanol gives ethylamines.
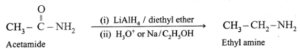
(v) Gabriel phthalimide synthesis :
This method is used for the synthesis of primary amine. It involves the following three stages.
- Formation of potassium salt of phthalimide from phthalimide on reaction with alcoholic potassium hydroxide.
- Formation of N-alkyl phthalimide from the potassium salt by reaction with alkyl halide.
- Alkaline hydrolysis of N-alkyl phthalimide to form the corresponding primary amine.

- Aromatic amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
(vi) By Hofmann degradation (Hofmann rearrangement / Hofmann bromamide degradation / Hofmann hypobromite degradation ) :
The conversion of amides into amines in the presence of bromine and alkali is known as Hofmann degradation of amides.
- An important characteristic of this reaction is that an amine with one carbon less than those in the amide is formed. Thus, decreasing the length of carbon chain.
- This reaction is an example of molecular rearrangement and involves the migration of an alkyl or aryl group from the carbonyl carbon to the adjacent nitrogen atom.
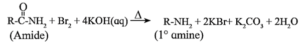
Examples :
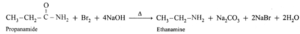
(a) When propanamide is treated with bromine and aqueous or alcoholic sodium hydroxide, ethanamine is obtained which has one carbon atom less.
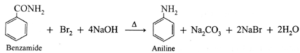
(b) When benzamide is treated with bromine and aqueous or alcoholic sodium hydroxide, aniline is obtained.
(c) When acetamide is treated with bromine and aq. or alcoholic solution of KOH, methyl amine is obtained, which has one carbon atom less. As the product contains
one carbon atom less than the original amide. It is a step down reaction.

Physical properties of Amines :
(i) Intermolecular forces, boiling points and solubility :
Primary and secondary amines have boiling points higher than the tertiary amines :
- The N-H bond in amines is polar in nature because of electronegativities of nitrogen (3.0) and hydrogen (2.1) are
- Due to the polar nature of N—H bond, primary and secondary have strong intermolecular hydrogen bonding.
- Tertiary amines do not have intermolecular hydrogen bonding as there is no hydrogen atom on nitrogen of tertiary amine.
- Thus, intermolecular forces of attraction are strongest in primary and secondary amines and weakest in to tertiary amines.
- Hence, primary and secondary amines have boiling points higher than the tertiary amines.
Amines have boiling points higher than the hydrocarbon but lower than the alcohols of comparable masses :
- Amines are polar than alkanes but less polar than alcohols.
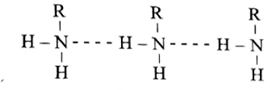
- Primary and secondary amines form intermolecular hydrogen bonds. This hydrogen bonding leads to an associated structure.
- The association is more in primary amines than that in secondary amines as there are two hydrogen atoms attached to the nitrogen atom.
- However, tertiary amines do not form intermolecular hydrogen bonds because they do not contain any hydrogen atoms attached to the nitrogen atom.
- Hence, amines have higher boiling points than the hydrocarbons but lower boiling points than the alcohols of comparable masses.
Intermolecular hydrogen bonds between the molecules of a primary amines
- The observed order of boiling points of isomeric amines is : primary amine > secondary amine > tertiary amine. It can be explained on the basis of the intermolecular forces in them.
- The lower aliphatic amines are gases with fishy odour, middle members are liquids and higher members are solids under ordinary temperature and pressure.
Solubility :
Aniline and other arylamines are usually colourless liquids but get coloured as they are easily oxidised by air. Due to their ability to form hydrogen bond with water molecule, lower aliphatic amines are soluble in water (see Fig.).
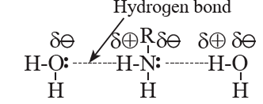
- Solubility of amines decreases with increase in molar mass of amines due to increase in size of hydrophobic alkyl group.
- Aromatic amines and higher aliphatic amines are insoluble in water.
- Since N-H bonds in amines are less polar than O-H bond in alcohol, water solubilities of alcohols, amines and alkanes of comparable molar mass in water are in the decreasing order: alcohols > amines > alkanes.
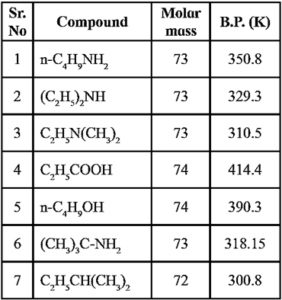
The order of boiling points of alkanes, amines, alcohols and carboxylic acid of comparable molar mass is as follows : Alkanes < Amines < Alcohols < Carboxylic acid. (see below table)
Boiling points of alkane, alcohol and amines of similar molar masses :
PDF : Chapter-13-Amines-Text Book
PDF : Chapter-13-Amines- Notes
PDF : Chapter-13-Amines- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF) Rs.137-Buy
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 12- Aldehydes, Ketones and Carboxylic acids – Online Notes
Next Chapter : Chapter-14-Biomolecules– Online Notes
We reply to valid query.