Elements of Group 1 and 2
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -8
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
The periodic table's first element is hydrogen. At the top of group 1 of the alkali metals is hydrogen. But it differs from alkali metals in a number of respects separately in the periodic table.
Hydrogen :
- Hydrogen exists in diatomic form as H2 molecule; therefore it is often called dihydrogen.
- A hydrogen atom is made up of an extra nuclear electron and a nucleus with +1 charge.
- It has a little tendency to lose this electron, but hydrogen may readily pair up with the other electron to form a covalent bond.
Occurrence :
- The primary component of the solar system is hydrogen.
- It accounts for roughly 70% of the universe's total mass.
- It is the third most abundant element on atom basis and is the tenth most plentiful element in terms of mass.
Position of hydrogen in the periodic table :
Hydrogen shows similarity with alkali metals :
- Hydrogen has electronic configuration is which is similar to outer electronic configuration of alkali metals, ns1.
- Both, hydrogen and Alkali metals form univalent positive ions (+1) by loss of 1 electron.
- Both combine with halogen to form halides.
H2 + Cl2 → 2HCl
2Na + Cl2 → 2NaCl.
Hydrogen show similarity with halogens :
- Halogens have outer electronic configuration ns2np5 which resembles that of hydrogen 1s1. Both by gaining one electron aquire the electronic configuration of nearest inert gas, ns2 (Hydrogen) ns2np6 (Halogens).
- Both form univalent negative ion (−1).
- Both react with metals.
2Na(s) + H2(g) \(\underrightarrow{Δ}\) 2NaH(s)
2Na + Cl2 \(\underrightarrow{Δ}\) 2NaCl
Know This :
|
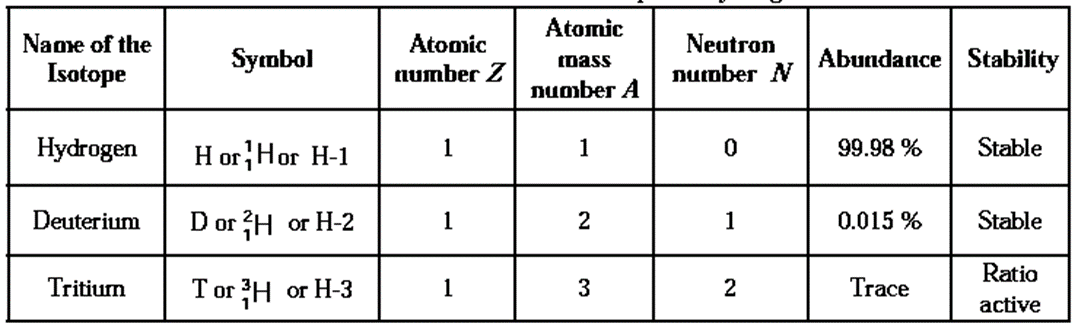
Isotopes of Hydrogen :
- If different atoms of the same element have different mass numbers they are called isotopes of each other.
- Hydrogen has three isotopes with mass numbers 1, 2 and 3.
- Hydrogen and Deuterium is stable. Tritium is Radioactive.

- They all contain one proton and one electron but different number of neutrons in the nucleus.
Characteristics of isotopes of hydrogen :
Preparation of dihydrogen
Hydrogen can be prepared using many methods.
(1) Laboratory methods :
The following are the laboratory methods for the preparation of hydrogen
(i) Dihydrogen is prepared in laboratory by the action of dilute hydrochloric acid on zinc granules.
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
(ii) Dihydrogen can be prepared by the action of aqueous solution of sodium hydroxide on zinc.
Zn(s) + 2NaOH(aq) → Na2ZnO2(aq) + H2(g)
(2) Industrial methods :
(i) By electrolysis of pure water:
- Since pure water is a poor conductor of electricity, it is acidified with dilute sulphuric acid to increase electrical conductivity.
- On a large scale dihydrogen is prepared by electrolysis of dilute sulphuric acid (acidified water) using platinum electrodes. Two volumes of dihydroen are liberated at cathode and one volume of oxygen is liberated at anode.
2H2O \(\frac{electrolysis}{traces\,\,of\,\,dilute\,\,H_2SO_4}>\) 2H2 ↑ + O2 ↑
(ii) From carbon or hydrocarbon: Three stages are involved in this industrial process of preparation of dihydrogen.
Stage 1 : Reaction of steam on coke or hydro- carbon in the presence of nickel catalyst, athigh temperature (1270 K) yields a mixture of carbon monoxide and

Stage II : Hydrogen is prepared from water gas by mixing it with steam and passing over iron chromate as catalyst (in a shift converter). Carbon monoxide in the watergas is transformed into carbon dioxide liberating H2 gas. This is called water gas shift reaction.
CO(g) + H2O(g) \(\frac{673\,\,K}{iron\,\,chromate}>\) CO2(g) + H2(g)
Stage III : Carbon dioxide is removed by scrubbing with sodium arsenite solution.
Obtaining pure dihydrogen :
Pure dihydrogen gas is obtained by electrolysis of warm solution of barium hydroxide using nickel electrodes. Dihydrogen obtained is about 99.5% pure.
Syngas :
- Syngas is the mixture of CO and H2. It is also called water gas.
- It is used for the synthesis of methanol and many hydrocarbons, hence, called syngas or synthesis gas.
- It is produced from saw dust or scrap wood.
- Production of syngas is the first stage of gastification of coal.
C(g) + H2O(g) \(\underrightarrow{1270\,\,K}\) CO(g) + H2(g)
Properties of dihydrogen
Physical properties :
- Dihydrogen is colourless, tasteless and odourless gas.
- It burns with a pale blue flame.
- It is a nonpolar water insoluble gas,
- It is lighter than air.
Chemical properties :
(i) Reaction with metals : Dihydrogen combines with all the reactive metals such as alkali metals, calcium, strontium and barium at high temperature, to form metal hydrides.
2Na(s) + H2(g) → 2NaH(s)
(ii) Reaction with dioxygen: Dihydrogen reacts with dioxygen in the presence of catalyst or by heating to form water. This reaction is highly exothermic.
2H2(g) + O2(g) \(\frac{catalyst}{heating}>\) 2H2O(l) ; ΔH = −235 kJ mol−1
(iii) Reaction with halogens: Dihydrogen reacts with fluorine at very low temperature and in dark at — 250 °C to form hydrogen fluoride, whereas it requires catalyst to react with iodine.
H2(g) + X2(g) → 2HX(g)
H2(g) + F2(g) \(\underrightarrow{-250^0C}\) 2HF
The vigour of reaction of dihydrogen decreases with increasing atomic number of halogen.
(iv) Reducing nature of dihydrogen :
Dihydrogen reduces oxides and ions of metals which are less reactive than iron to form corresponding metal, at moderate temperature.
CuO(s) + H2(g) → Cu(s) + H2O(l)
Fe3O4(s) + 4H2(g) → 3Fe(s) + 4 H2O(l)
Pd2+(aq) + H2(g) → Pd(s) + 2H+(aq)
(v) Hydrogenation :
The reaction in which hydrogen gas reacts with unsaturated organic compounds in the presence of a catalyst to form hydrogenated (saturated) compounds is called hydrogenation.
For example: Oil is converted to fats (vanaspati Ghee) using nickel catalyst.
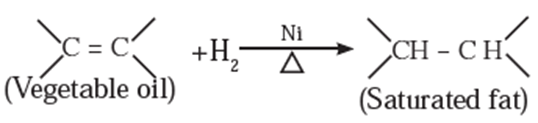
Hydroformylation of olefins : It give aldehydes which on further reduction give alcohols.
H2 + CO + R—CH=CH2 → R—CH2—CH2—CHO (Aldehyde)
H2 + R—CH2—CH2—CHO → R—CH2—CH2—CH2OH (Alcohol)
Uses of dihydrogen :
- Largest use of dihydrogen is in production of ammonia.
- Formation of vanaspati ghee by catalytic hydrogenation of oils.
- Liquid dihydrogen is used as a rocket fuel.
- Preparation of important organic compounds like methanol in bulk quantity.
2H2(g) + CO(g) \(\frac{cobalt}{catalyst}>\) CH3OH(l)
- Preparation of hydrogen chloride (HCl) and metal hydrides.
Alkali metals and alkaline earth metals :
Introduction :
The elements of the groups 1 and 2 are placed on the left in the periodic table. Here the last electron enters into ‘ns’ subshell. Thus they belong to the s-block of the periodic table.
s-block elements :
- The elements having atoms in which the last electron enters the outermost s-orbital are called s-block elements.
- The elements of s-block are normal elements, with general electronic configuration ns1 or 2.
- The s—block consists of two groups namly group 1 of alkali metals and group 2 of alkaline earth metals.
- Position of s-block is at the extreme left of the periodic table.

Occurrence of s-block elements :
Group 1 of alkali metals : Among alkali metals, sodium and potassium are the sixth and seventh most abundant elements in earth's crust. Francium is radioactive with a short half life period. Hence, it does not occur appreciably in nature.
Group 2 of alkaline earth metals : Among alkaline earth metals, magnesium and calcium are found abundantly in earth's crust. Radium is radioactive and rarest element.
Q. Why are the (i) elements of group 1 called alkali metals and (ii) elements of group 2 called alkaline earth metals?
(i) Group 1 of the periodic table consists of metals, Li, Na, K, Rb, Cs and Fr. Their oxides and hydroxides combine with water to form alkali, hence, they are called alkali metals. (ii) Group 2 of the periodic table consists of metals, Mg, Ca, Sr, Ba and Ra. These elements form alkaline oxides and hydroxides and they occur minerals in rocks.
Electronic configuration of elements of group 1 and group 2 Electronic configuration of group 1 elements : Electronic configuration of group 2 elements :


Trends in atomic and physical properties of elements of group 1 and group 2 :
Trends in atomic radii of alkali metals :
- Alkali metals have the largest atomic radii in their respective periods.
- In the group, as atomic number increases the valence electron enters a new shell hence atomic radii increase, from top to bottom.
- Thus, atomic radii increase in the order of Li < Na < K < Rb < Cs < Fr.
- Lithium has the smallest atom
Trends in ionization enthalpy of alkali metals :
- Ionization enthalpies of alkali metals are low in their respective periods due to their large atomic radii.
- It decreases down the group from lithium to caesium due to increase in atomic radii. Among the alkali metals, lithium has the highest ionization enthalpy.
Trends in atomic radii of group 2 elements :
- Atomic radii of alkaline earth metals are smaller than those of corresponding alkali metals in any period.
- Along the group, as atomic number increases, the valence electron enters a new shell, hence, atomic radii increase from Be to Ba.
Trends in ionization enthalpy of group 2 elements:
- Ionization potential of group 2 metals are high compared to alkali metals in any period.
- Down the group, as the atomic size increases the ionization enthalpy decreases from Be to Ba.
Properties of alkali metals and alkaline earth metals :
Alkali metals
Alkaline earth metals
All the alkali metals are silvery white and soft.
The alkaline earth are metals also in general silvery white lustrous and soft, but harder than the alkali metals.
Due to their large atomic size these elements have low density.
Density is higher than alkali metals
They are the most electropositive elements.
Comparatively less electropositive than the alkali metals.
Unipositive ions of all the elements of group 1 have inert gas configuration. Thus they have no unpaired electron and their compounds are diamagnetic and colourless.
The divalent ions of group 2 elements also have inert gas configuration with no unpaired electron, and therefore their compounds are also diamagnetic and colourless.
Chemical properties of elements of group 1 and group 2:
The elements of group 1 and group 2 both being s-block elements, show similarily in their chemical properties. The differences are due to variation in the atomic radii, ionization enthalpies and valencies.
- The alkali metals and alkaline earth metals are very reactive due to their low ionization enthalpy. As a result they are always found in combined state.
- Alkali metals due to their large atomic radii and low ionization enthalpy form univalent positive ions M+ easily. Therefore, they are more reactive than alkaline earth metals.
- Alkaline earth metals form bivalent positive ions M2+. The reactivity of these metal, increases down the group as the ionization enthalpy decreases in a group.
(i) Reaction with oxygen/air
All the elements of group 1 rapidly lose their luster in air due to formation of a layer of oxide, on peroxide and in some cases superoxide by reaction with oxygen in air.
- Lithium forms lithium monoxide : 2Li + O2 → 2LiO
- Sodium form sodium peroxide : 2Na + O2 → Na2O2
- Potassium form potassium superoxide : K + O2 → KO2
| Know This :
The reaction of Na and K with oxygen is highly exothermic and these metals catch fire when exposed to air. Potassium superoxide has ability to absorb carbon dioxide and give out oxygen at the same time: 4KO2 + 2CO2 → 2K2CO3 + 3O2 ↑ • This property of KO2 has been made use of in breathing equipment used for mountaineers and in submarines and space. |
Nature of oxides of group 1 metals :
The oxides of group 1 metals are strongly basic in nature. They dissolve in water forming aqueous solutions of strong alkali.
For example, Li2O(s) + H2O(l) → 2LiOH(aq)
Action of air (oxygen) on group 2 elements :
All the elements of group 2 burn when ignited with air forming ‘MO’ type of oxides and nitrides. For example,
2Mg + O2 → 2MgO
3Mg + N2 → Mg3N2
These metals are protected from further oxidation by the formation of oxide film on their surfaces. [Further heating of oxide forms peroxide].
(ii) Reaction with water
Group 1 :
- Lithium (Li), sodium (Na) and potassium (K) all float on water due to hydrogen bubbles released on reaction with water.
- Lithium reacts slowly but sodium and potassium react vigorously with water.
- Due to highly exothermic reaction sodium and potassium catch fire when put in water.
2Na + 2H2O → 2Na+ + 2OH− + H2 ↑
Group 2 :
- The elements of group 2 react with water to form metal hydroxide and hydrogen. Beryllium (Be) does not react with water.
- Magnesium (Mg) decomposes hot water, other elements react with cold water forming metal hydroxide M(OH)2 and hydrogen gas.
Ca + 2H2O → Ca(OH)2 ↓ + H2 ↑
(iii) Reaction with Hydrogen
- Group 1 : Alkali metals react with hydrogen at high temperature forming metal hydrides.
2M + H2 \(\underrightarrow{673\,\,K}\) 2M+H−
- For example, Sodium reacts with hydrogen at high temperature to form sodium hydride.
2Na + H2 \(\underrightarrow{Δ}\) 2NaH
- Group 2 : Except Be, the alkaline earth metals form hydrides at high temperature.
M + H2 \(\underrightarrow{Δ}\) MH2.
- For example, Calcium reacts with hydrogen to form calcium hydride.
Ca + H2 \(\underrightarrow{Δ}\) CaH2.
(iv) Reaction with Halogens
- Group 1 : All the alkali metals react vigorously with halogens to produce their ionic halide salts.
2M + X2 → 2M+X−
- Group 2: All the alkaline earth metals combine with halogens at high temperature to form halides.
M + X2 → MX2
- For Example Ca + Cl2 → CaCl2
(v) Reducing nature :
- Group 1 : Alkali metals have large atomic radii and low ionization enthalpy. Hence they lose their valence electron, ns1 easily. They are good reducing agents and have high negative value of standard reduction potentials. Lithium is the most powerful reducing agent.
- Group 2 : Alkaline earth metals lose ns2 electrons from their valency shell. They are good reducing agents but reducing power is less than that of alkali metals.
(vi) Reaction with liquid ammonia :
Group 1 : Alkali metals are soluble in liquid ammonia giving deep blue coloured solution which shows electrical conductivity.
M + (x + y)NH3 → [M(NH3)x]+ + [e(NH3)y]−
The ammonical electron gives deep bluecolour to the solution. The solution is paramagnetic and on standing liberates hydrogen with the formation of metal amide. With this, the blue colour changes to bronze and the solution becomes diamagnetic.
M+(am) + e−(am) + NH3(l) → MNH2 + H2(g)
[Note : (am) denotes solution in arnmonia.
[M(NH3)x]+ + [e(NH3)y]− Thus, these ions conduct electric current. Hence the solution shows electrical conductivity.]
Group 2 : The alkaline earth metals also dissolve in liquid ammonia which gives deep blue-black solution.
M + (x + 2y)NH3 → [M(NH3)x]2+ + 2[e(NH3)y]−
Diagonal Relationship :
The relative placement of elements with similar properties in the periodic table appears to be across a diagonal and is called diagonal relationship.
For example Li,
- Lithium is in group 1 and period 2.
- It resembles magnesium in group 2 and period 3 which is placed diagonally.
- This type of diagonal similarities is referred to as diagonal relationship.

The diagonal relationship is due to similarity in ionic sizes and charge / radius ratio of the elements.
For example, For Li and Mg,
Radii : Li = 152 pm Mg = 160 pm
Li+ = 76 pm Mg+ = 72 pm
Therefore, polarising power of Li+ and Mg+ are nearly same and electronegativities are not much different.
Some properties of Li and Mg, which indicate the diagonal relationship :
(i) Both lithium and magnesium form monoxide and nitride when heated in air.
4Li + O2 \(\underrightarrow{Δ}\) 2Li2O; 6Li + N2 \(\underrightarrow{Δ}\) 2Li3N.
2Mg + O2 \(\underrightarrow{Δ}\) 2MgO; 3Mg + N2 \(\underrightarrow{Δ}\) Mg3N2.
(ii) Lithium carbonate and magnesium carbonate decompose on heating to form their monoxides
Li2CO3 \(\underrightarrow{Δ}\) Li2O + CO2
MgCO3 \(\underrightarrow{Δ}\) MgO + CO2
Both, LiCl and MgCl2 crystallise from aqueous solutions as their hydrates. LiCl.2H2O and MgCl2.8H2O.
Q. How does lithium differ from other alkali metals?
Ans. Lithium differs from other alkali metals as follows : 4Li + O2 \(\underrightarrow{Δ}\) 2Li2O; Li + N2 \(\underrightarrow{Δ}\) 2Li3N. Li2CO3 \(\underrightarrow{Δ}\) Li2O + CO2
Diagonal relationship between beryllium and aluminium :

- Beryllium is in group 2 and period 2.
- It resembles aluminium in next main group 13 and period 3.
- This type of diagonal similarities are referred to as diagonal relationship.
Some properties of Be and Al, which indicate the diagonal relationship
Due to diagonal relationship Be resembles Al.
(1) Both, beryllium oxide and aluminium oxide are amphoteric. Thus they react with acid as well as alkali.
BeO + 2HCl(aq) → BeCl2 + H2O
BeO + 2NaOH(aq) → Na2BeO2 + H2O
Al2O3 + 6HCl(aq) → 2AlCl3 + 3 H2O
Al2O3 + 2NaOH(aq) → 2NaAlO2 + H2O
(2) Both, Be and Al from covalent chlorides. BeCl2 and AlCl3 are strong Lewis acids and are soluble in organic solvents.
BeCl2 has a chain structure with Cl bridges.
Aluminium Chloride dimer with Cl bridges

Q. How beryllium differs from other members of group 2?
BeO + 2HCl(aq) → BeCl2 + H2O MO + 2HCl → MCl2 + H2O BeO + 2NaOH(aq) → Na2BeO2 + H2O MO + NaOH → No reaction [M = other alkaline earth metals].
Uses of elements of group 1 and group 2 Group 1 :
Group 1:
- Lithium metal is used in long-life batteries used in digital watches, calculators and computers.
- Liquid sodium has been used for heat transfer in nuclear power station.
- Potassium chloride is used as a fertilizer.
- Potassium is used in manufacturing potassium superoxide (KO2) for oxygen generation. It is good absorbent of carbon dioxide.
- Caesium is used in photoelectric cells.
Group 2 :
- Beryllium is used as a moderator in nuclear reactors.
- Alloy of magnesium and aluminium is widely used as structural material and in aircrafts.
- Calcium ions are important ingredient in biological system, essential for healthy growth of bones and teeth.
- Barium sulphate is used in medicine as barium meal for intestinal x-ray.
- Radium is used in radiotherapy for cancer treatment.
Biological importance of elements of group 1 and group 2 : Group 1 : Group 2 :
| Know This :
Calcium has higher metallic character, greater tendency to lose valence electron and lower ionization enthalpy than magnesium. Therefore Mg reacts slowly with air, forming a thin film of oxide, resulting into tarnishing, whereas Ca reacts readily at room temperature with oxygen and nitrogen in the air. |
PDF : Class-11-Chemistry-Chapter-8- Elements of Group 1 and 2- Notes
PDF : Class-11-Chemistry-Chapter-8- Elements of Group 1 and 2-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-7-Modern Periodic Table – Online Notes
Next Chapter : Chapter-9-Elements of Group 13, 14 and 15 – Online Notes
We reply to valid query.