Amines
Maharashtra Board-Class-12-Chemistry-Chapter-13
Notes-Part-2
Topics to be Learn : Part-2
|
Basicity of Amines :
The basic nature of amines is due to presence of a lone pair of electrons on the nitrogen atom.
Lewis theory : Amines are bases because they can share a lone pair of electrons on ‘N’ atom with an electron deficient species.
Example : Trimethylamine shares its lone pair of electrons with the electron deficient boron trifluoride.
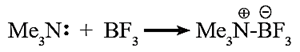
Basic strength of aliphatic amines :
Basic strength of amines is expressed quantitatively as Kb or pKb value. In terms of Lowry-Bronsted theory, the basic nature of amines is explained by writing the following equilibrium.
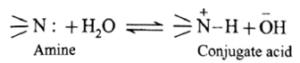
- In this equilibrium amine accepts H+, hence an amine is a Lowry-Bronsted base.
- The nitrogen atom in amines has a lone pair of electrons, which can be donated to suitable acceptor like proton H+.
- The aqueous solutions of amines are basic in nature due to release of free OH— ions in solutions. Hence amines are Lewis bases. There exists an equilibrium in their aqueous solutions as follows :
![]()
- Since OH— is a stronger base, equilibrium shifts towards left hand side giving less concentration of OH—.
- Here, Kb value is smaller and pKb value is larger. Hence amines are weak bases.
Order of basicity in ammonia and aliphatic amines :
- Since nitrogen atom in ammonia molecule has a lone pair of electrons, it is a Lewis base.
- Greater the availability of an electron pair, more is the basic character.
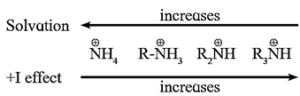
- Since alkyl group (R-) is an electron releasing group with (+1) inductive effect, alkyl amines act as a stronger base than ammonia.
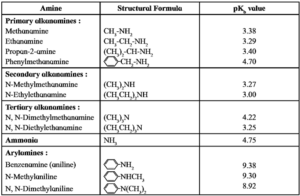
The trend in the observed pKb values (see table) and basic strength of 1°, 2°, 3° amines and NH3 can be represented as shown below :
Order of pKb values: NH3 > R-NH2 > R2NH < R3N
Order of basic strength : NH3 < R-NH2 < R2NH > R3N
- The availability of a lone pair of electrons on a nitrogen atom in amines is influenced by steric factor due to crowding of alkyl groups which affects solvation along with inductive effect of alkyl groups.
- Due to high energy of solvation of NH4+ ions, they acquire higher stability in aqueous solutions.
- The presence of alkyl groups in secondary and tertiary amines, due to steric hindrance decrease the solvation energy.
- This effect is more in tertiary amines making the tertiary ammonium ions (R3NH+) unstable as compared to secondary ammonium ion (R2NH2).
- Hence the cumulative effect on the order of basicity of amines is, secondary amine > primary amine > tertiary amine > ammonia (NH3).
pKb Values of some amines in aqueous medium :
Influence of +I effect :
The alkyl group in primary amines has + I effect i.e. (electron releasing).
![]()
The alkyl group tends to increase the electron density on the nitrogen atom. As a result, amines can donate the lone pair of electrons on nitrogen more easily than ammonia.
The amine being a base, can donate a pair of electrons to an acid. The alkyl group with + I effect will disperse the positive charge on the cation more than ammonia.

Due to + I effect of alkyl group cation formed by primary amine is more stable compared to cation formed from ammonia. Also it is seen that observed increasing basic strength from ammonia to primary amine is explained on the basis of increased stabilization of conjugate acids by + I effect for the presence of alkyl (R) groups. Hence, primary or aliphatic amine is a stronger base than ammonia.
Influence of solvation by water on stabilization of conjugate acids of aliphatic amines and ammonia can be represented as shown below :
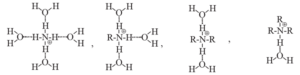
The solvent water stabilizes the conjugate acid by hydrogen bonding through the ‘H’ bonded to the ‘N+’. The number of ‘H’ atoms bonded to the ‘N+’ decreaes from 4 in NH4+ to 1 in R3NH+. As a result NH4+ is best stabilized by solvation while the stabilization by solvation is very poor in R3NH+.
Combined influence of +I effect and solvation on stabilization if conjugate acids of aliphatic amines decides the observed basic strength and pKb value. These two influencing factors operate in opposite directions.
The net results is that as we move from NH3 to RNH2 to R2NH, the basic strength increases due to better stabilization of the corresponding conjugate acids. But 3° amine is weaker base than 2° amine because the stabilization of conjugate acid of 3° amine by solvation is very poor.
(R3N) or tertiary amine or 3° amine is weaker base than secondary amine (R2NH) or 2° amine :
(i) Influence of +I effect : The increase in basic strength from 1° amine to 2° amine is explained on the basis of increased stabilization of conjugate acids by + I effect of increased number of alkyl group. However, decreased basic strength of 3° implies that the conjugate acid of 3° amine is less stabilized and is weak base though the + I effect of three alkyl groups in R3N+H is large.
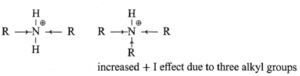
(ii) Influence of solvation :
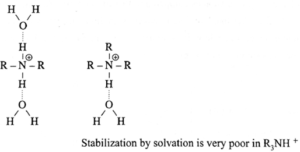
R2N+H is best stabilized by solvation while the stabilization by solvation is very poor in R3N+H. Hence (R3N) or tertiary amine or 3° amine is weaker base than secondary amine (R2NH) or 2° amine.
Basicity of arylamines :
From the pKb values we understand that arylamines in general are weaker bases than ammonia and aliphatic amines.
Strength of arylamines is explained in accordance with Lowery Bronsted theory by writing the following equilibrium.
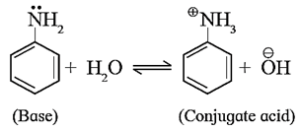
Here, both the species base and conjugate acid, are resonance stabilized but to different extent.
In arylamines, the -NH2 group is attached directly to an aromatic ring. The lone pair of electrons on nitrogen is conjugated to the aromatic ring and is less available for protonation.
Aniline is less basic than ammonia :
Less basic character of aniline can be explained on the basis of resonance shown by aniline.

- Due to resonance, the nitrogen atom of amino group in aniline acquires a positive charge, hence, lone pair of electrons is less available for protonation as compared to that of ammonia. Aniline is resonance stabilized by five resonance structures.
- On the other hand, aniline in agueous medium, accepts a proton does not have lone pair of electrons on nitrogen to produce a very low concentration of anilium ion and anilium ion shows only two resonance structures and therefore less stabilized than anline.
- Thus, aniline is more stable than anilium ion. Hence aniline accepts proton less readily or less basic in nature than ammonia.
Chemical properties of amines
(i) Laboratory test for amines :
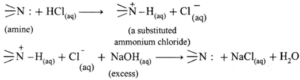
- Test for amines as the ‘base’ : All amines are basic compounds. Aqueous solution of water soluble amines turns red litmus blue.
- When water insoluble amine is dissolved in aqueous HCI, forms water soluble substituted ammonium chloride, further a substituted ammonium chloride on reaction with excess aqueous NaOH regenerates the original insoluble amine.
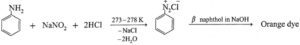
- Diazotization reaction / Orange dye test : In a sample of aromatic primary amine, 1—2 mL of conc. HCl is added. The aqueous solution of NaNO2 is added with cooling. This solution is transferred to a test tube containing solution of β-naphthol in NaOH. Formation of orange dye indicates presence of aromatic primary amino group. (It may be noted that temperature of all the solutions and reaction mixtures is maintained at 273 — 278 K throughout the reaction.)
(ii) Alkylation of amines : Hofmann’s exhaustive alkylation :
- Hofmann’s Exhaustive alkylation : When a primary amine is heated with excess of primary alkyl halide it gives a mixture of secondary amine, tertiary amine along with tetra alkylammonium halide.

- If excess of alkyl halide is used, tetraalkyl ammonium halide is obtained as major product. The reaction is known as exhaustive alkylation of amines.
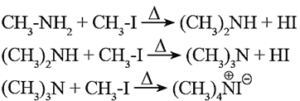
- Hofmann’s Exhaustive Methylation : The process of converting a primary, secondary or tertiary amine into quaternary ammonium halide by heating them with excess of methyl iodide, is called exhaustive methylation or Hofmann’s exhaustive methylation.
- Thus when methyl amine is heated with excess of methyl iodide it forms dimethylamine (secondary amine), then trimethylamine (a tertiary amine) and finally of quaternary ammonium iodide. The reaction is carried out in the presence of mild base NaHCO3, to neutralize the large quantity of HI formed.
Examples :
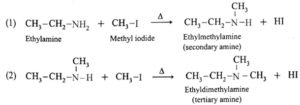
(a) Products of exhaustive methylation of Ethylamine.
A primary amine, ethylamine (CH3—CH2—NH2) on exhaustive methylation, i.e., on heating with excess methyl iodide, forms secondary amine, tertiary amine and finally a quaternary ammonium salt, ethyl-trimethyl ammonium iodide.
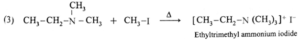
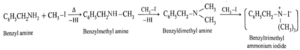
(b) Products of exhaustive methylation of Benzylamine.
Benzylamine C6H5CH2NH2 on exhaustive methylation i.e., on heating with excess methyl iodide forms benzylmethyl amine, benzyldimethyl ammonium chloride and finally benzyltrimethyl ammonium iodide.
(iii) Hofmann Elimination :
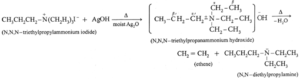
- When tetra alkyl ammonium halide is heated with moist silver hydroxide, a quaternary ammonium hydroxide is obtained. Quaternary ammonium hydroxides are deliquescent crystalline solids and are basic in nature.
- Quaternary ammonium hydroxides on strong heating undergo f-elimination to give tertiaryamine, alkenes and water, the reaction is called Hofmann elimination. The major product is least substituted alkene.
Example :
Know This :
CH3-CO-O-CH2-CH2-N⊕(CH3)3
|
(iv) Acylation of amines :
- The reaction of amines with acetyl chloride (cthanoyl chloride) is called acetylation of amines.
- Aliphatic and aromatic primary and secondary amines undergo acylation reaction. These amines contain replaceable hydrogen atoms (positively polarised H) on the nitrogen atom. These hydrogen atoms are replaced by acyl groups such as acetyl group.
- Acylation is a nucleophilic substitution reaction. The reaction is carried out in presence of strong base like pyridine, which neutralizes the acid produced during the reaction.
Examples :
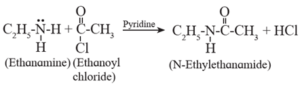
(a) Acetyl chloride ( ethanoyl chloride) on reaction with ethylamine forms monoacetyl derivative, N-ethylacetamide (or N-acetyl ethylamine).
(b) Diethyl amine on reaction with acetyl chloride (ethanoyl chloride) forms N-acetyl dimethylamine.

(c) Triethyl amine, being a tertiary amine does not have H atom attached to nitrogen of amine, hence it does not react with acetyl chloride (ethanoyl chloride).

(v) Carbylamine reaction :
Aliphatic or aromatic primary amines on heating with chloroform and alcoholic potassium hydroxide solution form carbyl amines or alkyl/aryl isocyanides with extremely unpleasant (foul) smell. This reaction is a test for primary amines. Secondary and tertiary amines do not give this test.
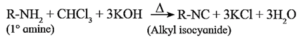
Example :
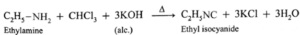
(vi) Reaction with nitrous acid :
- Aliphatic primary amines on reaction with nitrous acid form aliphatic diazonium salts as very unstable intermediates which decompose immediately by reaction with solvent water.
- Corresponding alcohol is formed as the product of the reaction and nitrogen gas is liberated.

Example :
Ethyl amine on reaction with nitrous acid in cold forms aliphatic diazonium salt, (unstable intermediate), which decomposes immediately by reaction with solvent water to produce ethyl alcohol and nitrogen gas.

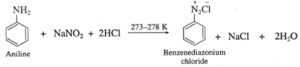
- Aromatic primary amines react with nitrous acid to form diazonium salts which have reasonable stability at 273 K.
- The conversion of primary aromatic amine into diazonium salts is called diazotisation.
Example :
(a) Aniline reacts with nitrous acid in cold to form diazonium salt which has reasonable solubility at 273 K.

(b) Benzenediazonium chloride is prepared by the action of nitrous acid on aniline at 273-278 K. Nitrous acid being unstable, is prepared in situ by the reaction between sodium nitrite and dilute hydrochloric acid.
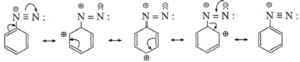
- Aryl diazonium salts are resonance stabilized and useful as versatile intermediates to obtain a variety of products.
Resonance stabilized structures of aryl diazonium salt :
Reactions of arene diazonium salts:
Aryl diazonium salts show two types of reactions.
(i) Reactions involving displacement of diazo group :
Reactions involving replacement of dlazomum group by nucleophiles such as Cl—, Br—, I—, F—, CN—, H—, OH—, etc. Nitrogen from diazonium group is lost as N2 gas.
(a) [Replacement by Cl—, Br—, and —CN : Sandmeyer reaction.] Freshly prepared aromatic diazonium salt on reaction with cuprous chloride gives aryl chloride, on reaction with cuprous bromide gives aryl bromide and on reaction with cuprous cyanide give aryl cyanide. The reaction in which copper (I) salts are used to replace nitrogen in diazonium salt is called Sandmeyer reaction.
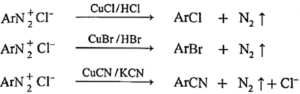
(b) Gattermann reactions :
The aryl chloride or bromides can also be prepared by Gattermann reactions in which diazonium salt reacts with Cu/HCl or Cu/HBr respectively.
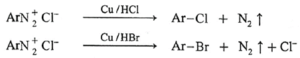
(c) Iodoarene formation :
When diazonium salt is warmed with potassium iodide, aryl iodide is obtained.
![]()
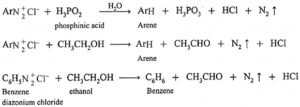
(d) Mild Reduction :
Arene diazonium salt on treatment with mild reducing agents like phosphinic acid (hypophosphoric acid) or ethanol, arene is obtained.
(e) Phenol formation :
When arene diazonium salt is slowly added to a large volume of boiling dilute sulphuric acid, phenol is obtained.
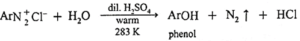
(f) Reaction with fluoroboric acid :
When fluoroboric acid is treated with the solution of diazonium salt, a precipitate of diazonium fluoroborate is obtained, which is filtered and dried. When dry diazonium fluoroborate is heated, it decomposes to give aryl fluoride. This reaction is called Balz-Schiemann reaction.
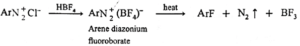
Examples :
When benzene diazonium fluoroborate is heated with aqueous solution of sodium nitrite in the presence of copper powder nitrobenzene is obtained.

Benzene diazonium fluorobate can be obtained by reaction of benzene diazonium chloride with HBF4
![]()
(ii) Reactions involving retention of diazo group: (Coupling reactions) :
- Diazonium salts react with certain aromatic compounds having an electron-rich group (e.g.—OH, —NH2, etc.) to form azo compounds. This reaction is an electrophilic substitution and is called coupling reaction.
- Azo compounds are brightly coloured and are used as dyes and indicators. Coupling reaction is an electrophilic substitution reaction.
Examples :
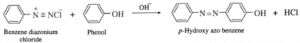
(a) Benzene diazonium chloride reacts with alkaline solution of phenol to give p-hydroxy azo benzene (orange dye).
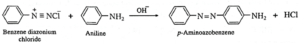
(b) Benzene diazonium chloride reacts with aniline in mild alkaline medium to give p-aminobenzene (yellow dye).
| Know This :
The acid-base indicator methyl orange is an azo dye.
|
Reaction with arenesulfonyl chloride :
Hinsberg’s test :
- Benzenesulfonyl chloride (C6H5SO2Cl) is known as Hinsberg’s reagent.
- This reaction is useful for the distinction of primary, secondary and tertiary amines.
(a) Primary amine (like ethyl amine) is treated with Hinsberg’s reagent (benzene sulphonyl chloride) forms N-alkyl benzene sulphonamide which dissolve in aqueous KOH solution to form a clear solution of potassium salt and upon acidification gives insoluble N-alkyl benzene sulphonamide.
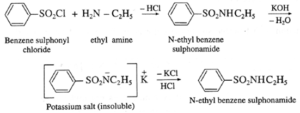
(b) Secondary amine like diethyl amine is treated with benzene sulphonyl chloride forms N,N-diethyl benzene which sulphonyl amide remains insoluble in aqueous KOH and does not dissolve in acid.
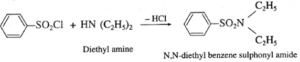
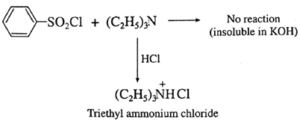
(b) Tertiary amine like triethyl amine does not react with benzene sulphonyl chloride and remains insoluble in KOH, however it dissolves in dil. HCl to give a clear solution due to formation of ammonium salt.
Examples :
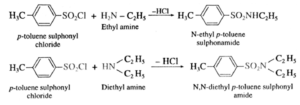
Action of p-toluene sulphonyl chloride on ethyl amine and diethyl amine :
Electrophilic aromatic substitution in aromatic amines :
Amino group is ortho and para directing and powerful ring activating group. As a result aromtic amines readily undergo electrophilic substitution reactions.
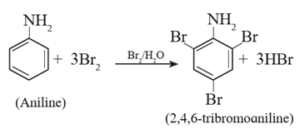
(i) Bromination :
Aniline reacts with bromine water at room temperature to give a white precipitate of 2,4,6- tribromoaniline.
(ii) Nitration : When aniline is warmed with a mixture of conc. nitric acid and conc. sulphuric acid (a nitrating mixture), a mixture of ortho, meta and para nitroaniline is obtained.
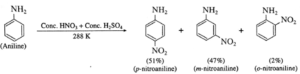
However, to get p-nitroaniline as major product, -NH2 group is first protected by acetylation, nitration is carried out and then amide is hydrolysed.

(iii) Sulfonation :
Aniline on treatment with cold sulphuric acid forms anilium hydrogen sulphate which on heating with sulphuric acid at 453 K — 475 K gives sulphanilic acid. (p-aminobenzene sulphonic acid) as major product.

Sulphanilic acid exists as a salt, called dipolar ion or zwitter ion. It is produced by the reaction between an acidic group and a basic group present in the same molecule.
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 12- Aldehydes, Ketones and Carboxylic acids – Online Notes
Next Chapter : Chapter-14-Biomolecules– Online Notes
We reply to valid query.