Elements of Groups 16, 17, and 18
Maharashtra Board-Class-12-Chemistry-Chapter-7
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
- P-block elements are those in which the differentiating electron—the final filling electron—enters the p-orbital of the atoms' outermost shell.
- The elements of groups 16, l7 and 18 are p-block elements.
- The p-sub shell can hold up to 6 electrons at a time, creating a maximum of 6 groups. P-block elements are found in groups 13 to 18 of the periodic table.
- The general electronic configuration of valence shell of p-block elements is ns2 np1−6 (except element He which has electronic configuration 1s2)
Factors influencing the properties of p-block elements :
- Atomic and ionic radii
- Ionisation enthalpy
- Electron gain enthalpy (or electron affinity)
- Electronegativity
- The presence or absence of electrons in d or d and f orbitals in the p-block elements.
Symbols and atomic numbers of p-block elements :
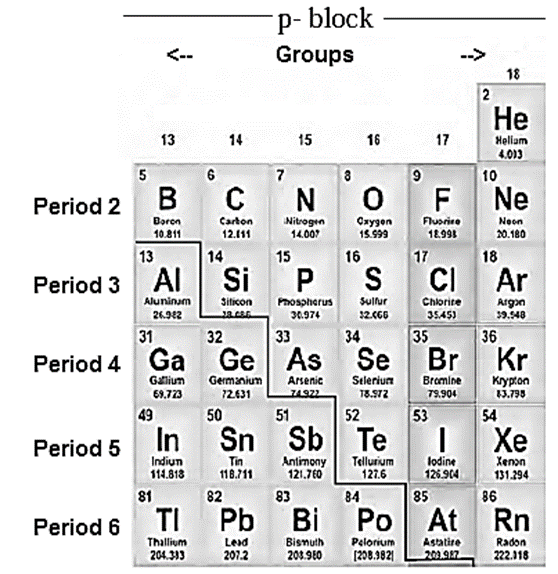
Occurrence :
Elements in groups 16, 17 and 18 :
Group 16 elements : Oxygen (8O ); Sulphur (16S); Selenium (34Se); Tellurium (52Te); Polonium (84Po)
- The group 16 is called oxygen family.
- Group 16 elements are called chalcogens or ore forming elements, since a large number of metal ores are present as oxides or sulphides.
- Oxygen : It is the most abundant of all the elements on earth. Oxygen forms 20.95 % by volume of air and about 46.6 % by mass of earth's crust.
- Sulfur : Sulfur forms 0.034% by mass of the earths crust. It occurs mainly in combined forms such as Sulfates and Sulfides as given below :
- Sulfates : Gypsum (CaSO4.2H2O), epsom salt (MgSO4.7H2O), baryte (BaSO4)
- Sulfides : Galena (PbS), zinc blende (ZnS), copper pyrites (CuFeS2).
- Selenium and tellurium : Selenium and tellurium are found as metal selenides and tellurides in sulphide ores.
- Polonium : Polonium occurs in nature as a decay product of radioactive thorium and uranium metals.
Group 17 elements : Fluorine (9F); Chlorine (17Cl); Bromine (35Br); Iodine (53I); Astatine (85At).
- Group 17 elements are collectively called halogens. In Greek, halo means salt and gene means born, so halogens are salt producing elements.
- Halogens are highly reactive due to high electro-negativities, hence do not occur in the free state.
- Group 17 elements occur mostly in the form of compounds.
- Fluorine is available as insoluble fluorides in the earth’s crust.
- The important minerals are fluorspar, CaF2, cxyolite, Na3AlF6, fluorapatite, 3Ca3(PO4 )2.CaF2.
- Halides (chloride, bromides, iodides) of Na, K, Mg and Ca are present in sea water.
- The dried up sea beds contain sodium chloride and the mineral carnallite (KCl.MgCl2.6H2O).
- Seaweeds contain up to 0.5% of iodine. The compound chile saltpetre contains 0.2% of sodium iodate.
- Astatine is the last member of the halogen family. It is radioactive and has a half life of 8.1 hours.
Group 18 elements : Helium (2He); Neon (10Ne ); Argon (18Ar); Krypton (36Kr); Xenon (54Xe); Radon ( 86Rn)
- All the noble gases except radon occur in the atmosphere.
- Their abundance in dry air is ∼ 1% (by volume) with argon as the major constituent.
- The main commercial source of helium is natural gas.
- Helium and neon are found in minerals of radioactive origin e.g. pitchblende, monazite, cleveite.
- Xenon and radon are the rarest elements of the group.
- Radon is a decay product of 226Ra.
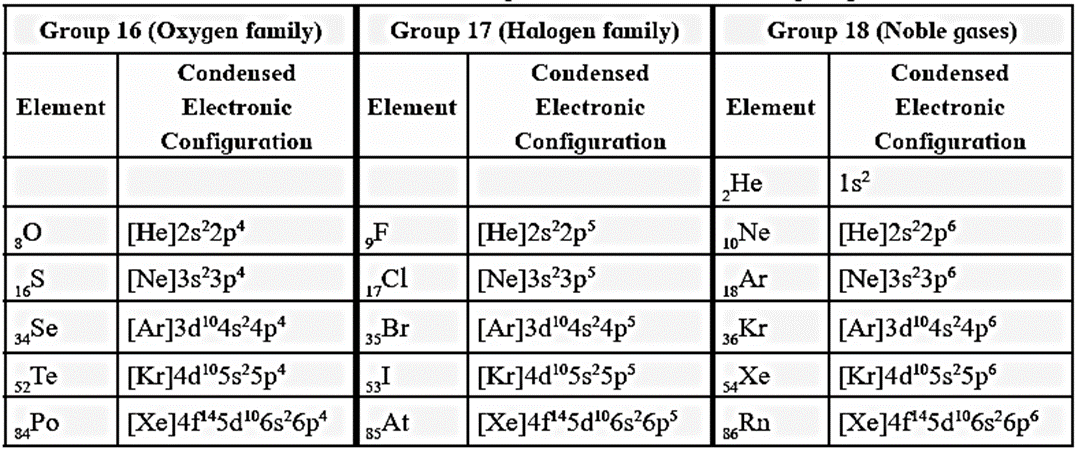
Electronic configuration of elements of group 16, 17 and 18:
- The general electronic configuration of group 16 elements is ns2np4, group 17 is ns2np5 and group 18 is ns2np6.
- Group l6 has two electrons less than the stable electronic configuration of the nearest noble gas, while group 17 has one electron less than the stable electronic configuration of the nearest noble gas.
Condensed electronic configuration of elements of group 16, 17 and 18 :
Atomic and physical properties of elements of group 16, 17 and 18.
Atomic and physical properties of group 16 elements :
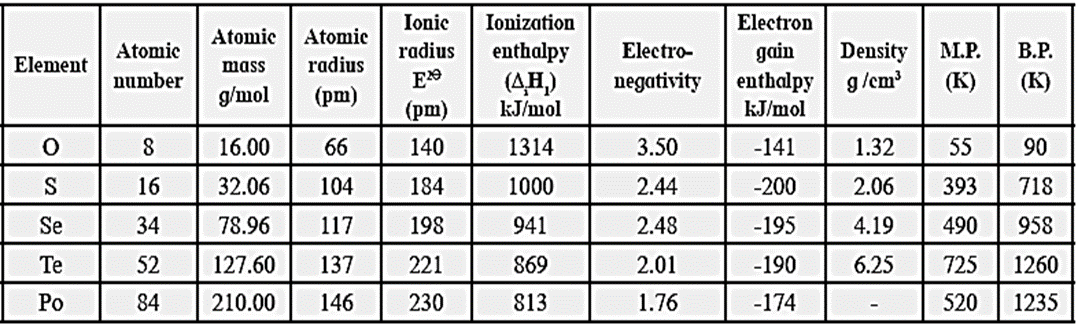
Trend in the atomic properties of group 16 elements :
(i) Atomic and ionic radii :
- As compared to group 15 elements, the atomic and ionic radii of group 16 elements are smaller due to higher nuclear charge.
- The atomic and ionic radii increase clown the group from oxygen to polonium. This is due to the addition of a new shell at each successive elements on moving down the group.
- The atomic radii increases in the order O<S<Se<Te<Po
(ii) Ionisation enthalpy :
- The ionisation enthalpy of group 16 elements has quite high values.
- Ionisation enthalpy decreases down the group from oxygen to polonium. This is due to the increase in atomic volume down the group.
- The first ionisation enthalpy of the lighter elements of group 16 (O, S, Se) have lower values than those of group 15 elements in the corresponding periods. This is due to difference in their electronic configurations.
Group 15 : (valence shell) ns2 npx1 npy1 npz1
Group 16 : (valence shell) ns2 npx2 npy1 npz1
- Group 15 elements have extra stability of half filled and more symmetrical orbitals, while group 16 elements acquire extra stability by losing one of paired electrons from npx-orbital forming half filled p-orbitals.
- Hence group 16 elements have lower first ionisation enthalpy than group 15 elements.
(iii) Electronegativity:
- The electronegativity values of group 16 elements have higher values than corresponding group 15 elements in the same periods.
- Oxygen is the second most electronegative elements after fluorine. (O = 3.5, F = 4)
- On moving down the group electronegativity decreases from oxygen to polonium.
- On moving down the group atomic size increases, hence nuclear attraction decreases, therefore electro-negativity decreases.
(iv) Electron gain enthalpy :
- Electron gain enthalpy (or electron affinity) is the energy released when an electron is added to the valence shell of a gaseous atom forming gaseous ion.
M(g) + e— → M(g) + energy
- More the energy released, more is electron gain enthalpy or electron affinity.
- Group 16 elements have high values for electron gain enthalpy. On moving down the group, electron gain enthalpy decreases from S to Po.
- Oxygen has less negative electron gain enthalpy, due to high electronegativity, low atomic size and high electron density so that the incoming election is repelled.
- Electron gain enthalpy of the elements decreases down the group due to successive decrease in electronegativity and nuclear attraction and increase in atomic size.
Trend in the density, melting and boiling points of Group 16 elements :
(i) Density :
- The density of group 16 elements increases down the group.
- On moving down the group, the increase in atomic mass is more than the increase in atomic size.
- Down the group the magnitude of van der Waals forces of attraction increases resulting in compact lattice formation of elements. Therefore the density increases down the group. The density increases in the order O < S < Se < Te
(ii) Melting and boiling point :
- The melting and boiling points of the elements increase regularly on moving down the group.
- However, the melting and boiling points of polonium are lower than that of tellurium. This is because the intermolecular van der Waals forces are weaker in polonium.
- There is also a large difference in the melting and boiling points of oxygen and sulphur as oxygen has a smaller size, while sulphur has a larger size and stronger van der Waals forces.
Atomic and physical properties of group 17 elements :
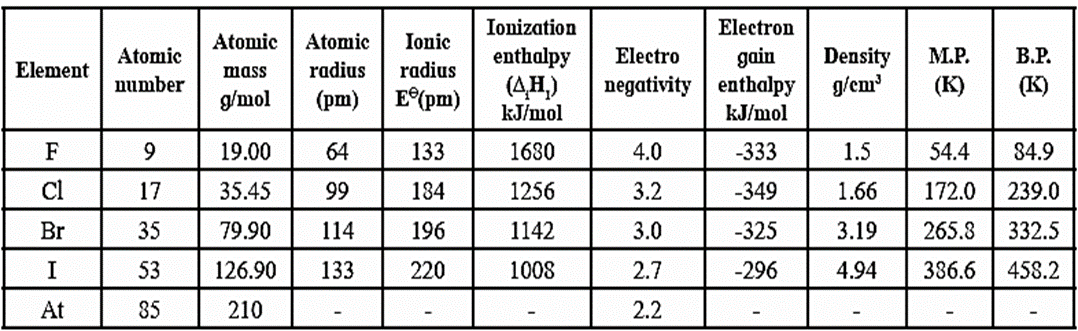
Trend in the atomic properties of group 17 elements :
(i) Atomic size :
- Atomic and ionic radii increase down the group as atomic number increases due to the addition of new electronic valence shell to each succeeding element.
- The atomic radii increase in the order F < Cl < Br < I
- Halogens possess the smallest atomic and ionic radii in their respective periods since the effective nuclear charge experienced by valence electrons in halogen atoms is the highest.
(ii) Ionisation enthalpy :
- The ionisation enthalpies of halogens are very high due to their small size and large nuclear attraction.
- The ionisation ethalpies decrease down the group since the atomic size increases.
- The ionisation enthalpy decreases in the order F > Cl > Br > I.
- Among halogens fluorine has the highest ionisation enthalpy due to its smallest size.
(iii) Electronegativity :
- Halogens have the highest values for electro-negativity due to their small atomic radii and high effective nuclear charge.
- Each halogen is the most electronegative element of its period.
- Fluorine has the highest electronegativity as compared to any element in the periodic table.
- The electronegativity decreases as, F > Cl > Br > I
(iv) Electron gain enthalpy (ΔegH) :
- The halogens have the highest negative values for electron gain enthalpy.
- Electron gain enthalpies of halogens are negative indicating release of energy.
- Halogens liberate maximum heat by gain of electron as compared to other elements.
- Since halogens have outer valence electronic configuration, ns2 np5, they have strong tendency to accept an electron to complete an octet and acquire electronic configuration of the nearest inert elements.
- In case of fluorine due to small size of 2 p-orbitals and high electron density, F has less negative electron gain enthalpy than Cl.
F(g) + e— → F—(g) ....ΔegH = — 333 kJ mol—1
Clog) + e— → Cl—(g) ......ΔegH = — 349 kJ mol—1
- The variation in electron gain enthalpy is inthe order off Cl > F > Br > I.
Trend in the density, melting and boiling points of Group 17 elements :
Density :
- Down the group, density of halogens increases.
- This is, because down the group, van der Waals forces of intermolecular attraction increase, and hence tendency for agglomerisation increases. Therefore density increases.
Melting point and boiling point :
- Halogens have low melting points and boiling points.
- Melting points and boiling points increase down the group.
Atomic and physical properties of group 18 elements :
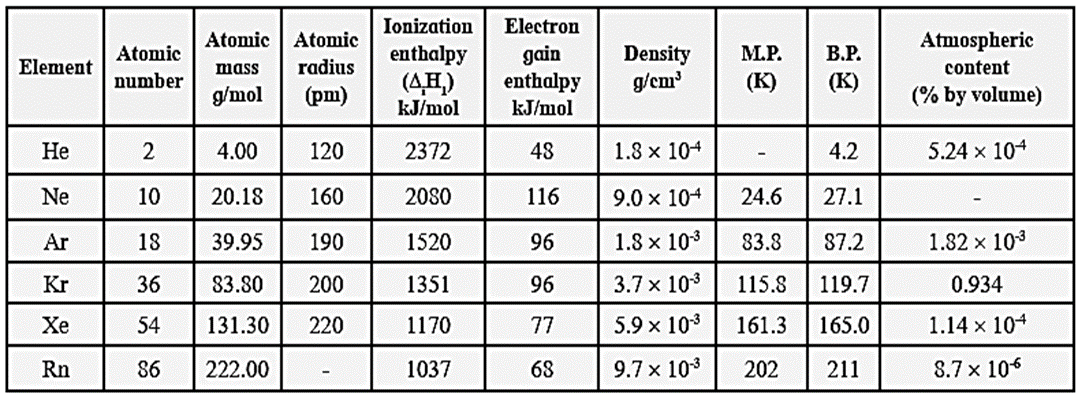
Trend in the atomic properties of group 18 elements :
(i) Atomic size :
- The atomic radii of group 18 elements is larger than the atomic radii of group 17 elements.
- Down the group from He to Xe, atomic radii increases due to increase in the number of quantum shells.
- The atomic radii increase in the order He<Ne<Ar<Kr<Xe
(ii) Ionisation enthalpy :
- In general, group 18 elements have high values of ionisation enthalpy.
- In a period, each noble gas has the highest ionisation enthalpy.
- The noble gases have electronic configuration, ns2 np6, they have complete octet with paired electrons and very stable closed shell electronic configuration. Therefore high energy is required to remove the electron from valence shell.
- Down the group ionisation enthalpy decreases.
(iii) Electron gain enthalpy :
- Group 18 elements have electronic configuration ns2 np6 and complete octet of electrons, hence they have no tendency to accept electrons.
- Therefore, they have a large positive electron gain enthalpy.
Trend in melting and boiling points of Group 18 elements :
Melting points and boiling points :
- Group 18 elements have very low melting points and boiling points.
- The melting points and boiling points increase down the group from He to Rn.
- As the size of the atoms increases on moving down the group, the magnitude of the van der Waals forces increase from He to Rn. Thus, the melting and boiling points increase from He to Rn.
- Helium has the lowest boiling point (4.2 K) of any known substance.
- The boiling points increase in the order He<Ne<Ar<Kr<Xe<Rn
Trend in the atomic properties of group 16, 17 and 18 :
(i) Atomic and Ionic radii
- In group 16, 17 and 18 atomic and ionic radii increase down the group, as a result of increase in the number of quantum shells.
- Across a period atomic or ionic radii decrease with increasing atomic number, consequent to increase in (Zeff) effective nuclear charge.
- Group 17 elements (Halogens) have the smallest atomic radii in their respective periods.
(ii) Ionisation enthalpy :
- The group 16, 17 and 18 elements have high ionisation enthalpy.
- The ionisation enthalpy decreases down the group due to increase in the atomic size.
- Across a period ionisation enthalpy increases with increase of atomic number. This is due to addition of electrons in the same shell.
- However the elements of group 16 have lower ionisation enthalpy values compared to those of group 15 in the corresponding periods, owing to extra stable half filled electronic configuration of p-orbitals in elements of group 15.
(iii) Electronegativity :
- In a group (16, 17 and 18) the electronegativity decreases down the group.
- Oxygen has the highest electronegativity next to fluorine amongst all the elements.
- Halogens have very high electronegativity. Fluorine is the most electronegative element in the periodic table.
(iv) Electron gain enthalpy :
- In the groups 16 and 17 electron gain enthalpy becomes less negative down the group.
- However in group 16, oxygen has less negative electron gain enthalpy than sulfur due to its small atomic size.
- In group 17, fluorine has less negative electron gain enthalpy than that of chlorine. This is due to small size of fluorine atom.
- Group 18 elements (noble gases) have no tendency to accept electrons because of their stable electronic configuration (ns2np6) and thus have large positive electron gain enthalpy.
Physical states and the types of elements of Groups 16, 17 and 18 :
Physical states :
- Oxygen is a gas, while the other elements of group 16 are solids at room temperature.
- All the elements of group 16 show allotropy and exist in several allotropic modifications.
- In group l7, fluorine and chlorine are gases, bromine is a liquid and iodine is a solid at room temperature.
- All halogens are coloured. Fluorine is a yellow coloured gas, chlorine is a greenish yellow coloured gas, bromine is a red coloured liquid and iodine is a violet coloured solid.
- All the elements of group 18 are monoatomic gases.
Types of elements :
- In group l6 elements, oxygen and sulphur are non-metals, selenium and tellurium are metalloids, while polonium is a radioactive metal with half life 138 days.
- All halogens are non-metals.
- Group 18 elements are chemically inert and hence called inert elements.
Anamalous Behaviour
Anomalous behaviour of oxygen :
Oxygen is the first element of group 16.
Reasons for anomalous behaviour of oxygen :
- It has the smallest size in the group.
- It has the highest electronegativity (3.5).
- It does not have vacant d-orbitals like other elements in group 16.
Oxygen shows following anomalous behaviour :
- Physical state ; Oxygen is a gas while other elements in the group are solids at ordinary temperature.
- Atomicity : Oxygen exists as a diatomic molecule O2 while other elements are polyatomic molecules like S8, Se8 have puckered ring structure.
- Magnetic behaviour: Molecular oxygen O2 is paramagnetic while other elements are diamagnetic. Molecular O2 has two unpaired electrons in the antibonding molecular orbitals.
- Oxidation states : Oxygen shows oxidation state −2 in oxides, −1 in peroxides while +2 in oxygen difluoride, OF2. Since it does not have vacant d-orbital it doesn’t show higher oxidation states while other elements of group 16 Show + 2, + 4 and + 6 oxidation states.
- Hydrogen bonding : Since Oxygen has high electronegativity (3.5), it forms hydrogen bonding in its compounds like H2O, alcohols, etc. Other elements in the group do not show this property.
- Hydrides ; The hydride of oxygen, H2O is a liquid while the hydrides of all other elements in group 16 are gases.
- Covalency ; Oxygen shows a common covalency 2 since it has only two unpaired electrons and no d -orbitals in its valence shell. In rare cases, it shows covalency 4. The other members of Group 16 can show covalency, more than 4, due to the presence of d-orbitals in their valence shell.
Anomalous behaviour of fluorine :
Fluorine, the first member of group 17, differs in properties from the other members of the group. The anomalous behaviour of fluorine is due to the following reasons.
- small atomic size
- high electronegativity
- absence of d-orbitals in valence shell
- low F-F bond dissociation enthalpy
Anomalous properties of fluorine :
The anomalous properties of fluorine are as follows :
- Fluorine has the highest reactivity among other halogens.
- Hydrogen bonding is present in HF only, while it is absent in other haloacids (HCl, HBr and HI).
- HF is a liquid while other hydrogen halides are gases at room temperature.
- HF is a weak acid while other haloacids are strong acids.
- Fluorine shows only one oxidation state −1 while all other halogens show variable oxidation states like −1, +1, +3, +5and +7.
- Fluorine has the highest electronegativity but less negative electron gain enthalpy than chlorine.
- The compounds of fluorine have higher ionic character than other halogens.
- Fluorine has no tendency to form polyhalide ion whereas other halogens form polyhalide ions like, Cl3−, Br3− and I3−
- Fluorine unlike other halogens when reacts with water and produces O2 and O3.
2F2 + 2H2O → 4HF + O2
3F2 + 3H2O → 6HF + O3
- Fluorine shows much higher values of ionization enthalpy, electronegativity and standard electrode potentials compared to the other halogens.
- Fluorine shows much lower values of ionic and covalent radii, melting and boiling points and electron gain enthalpy than expected.
- Fluorine forms only one oxoacid HOF, while the other halogens form a number of oxoacids.
Chemical Properties of elements of groups 16, 17 and 18 :
Oxidation state :
(i) The group 16 elements :
- The group 16 elements have the valence shell electronic configuration., ns2 np4
- They show variable oxidation states, − 2, + 2, + 4 and − 6.
- Since all elements have 6 valence electrons, they tend to gain or share 2 electrons to complete an octet, and show common oxidation state − 2.
- Oxygen being highly electronegative, it shows the common oxidation state − 2 in oxides (H2O, MgO). It shows − 1 oxidation state in peroxides (H2O2, Na2O2) and + 2 oxidation states in OF2.
- Except oxygen all other elements have vacant d-orbitals, hence they show higher oxidation states + 4 and + 6. For example, SF4 (4+), TeCl4 (4+), SeBr4 (4+), SP6 (6+), etc.
- The stability of the + 6 oxidation state decreases but the stability of the + 4 oxidation state increases down the group due to the inert pair effect.
- Bonding in + 4 and + 6 oxidation states is covalent in nature.
The group 17 elements :
- Group 17 elements are represented by their valence shell electronic configuration as ns2np5.
- They attain noble gas configuration either by gaining one electron forming e− ions or by sharing one electron forming one covalent bond with configuration ns2np6.
- Hence they are monovalent and show oxidation state −1.
- Since fluorine does not have vacant d-orbital, it shows only one oxidation state of −1 while all other halogens show variable oxidation states from −1 to +7. These oxidation states arc. −1, +1, + 3, + 5 and + 7. Cl and Br also show oxidation states + 4 and + 6 in their oxides and oxyacids.
The group 18 elements :
- The group 18 elements have a stable electronic configuration ns2np6 with completely filled orbitals.
- Due to completely filled orbitals and complete octets these elements do not show a tendency to lose, gain or share electrons.
- They have zero valency and mostly exist as monoatomic gases.
- Xenon exhibits higher oxidation states, as the paired electrons of the valence shell can be unpaired and promoted to the empty d-orbitals.
- The unpaired electrons are shared with fluorine or oxygen atoms to form covalent compounds with higher oxidation states such as XeF2, XeF4, XeF6, XeO3 and XeOF6.
Q. Why does xenon being a noble gas form compounds with other elements ?
Xenon forms compounds with other elements due to the following reasons :
- Xenon has large atomic size and lower ionization enthalpy.
- The paired electrons of the valence shell can be unpaired and promoted to the empty d-orbitals.
- The unpaired electrons are shared with fluorine or oxygen atoms to form covalent compounds with higher oxidation states.
Chemical Reactivity towards hydrogen:
(i) Group 16 elements :
- All the elements of group 16 react with hydrogen and form hydrides of the type H2M where M = O, S, Se, Te and Po. For example H2O, H2S, H2Se, H2Te and H2Po.
- The hydrides of these elements show regular trends in their physical and chemical properties.
- All these hydrides are formed by sp3 hybridisation of the central atom and have angular shape.
- All hydrides have similar structure but differ in H —M —H bond angle. This bond angle decreases from H2O to H2Te.
- All these hydrides have two M—H bond pairs and two lone pairs of electrons.
- Due to repulsion between lone pairs-lone pairs and lone pairs-bond pairs, and bond pairs - bond pairs the bond angle is reduced from regular 109° 28’.
- On moving down the group, atomic size increases, electronegativity decreases, electron density decreases.
- Since among group 16 elements, oxygen has the highest electronegativity, lowest atomic size and hence the highest electron density, the repulsion between electron pairs is maximum in H2O, hence H—O—H bond angle is maximum.
- The electron pair repulsion decreases down the group, hence bond angle also decreases down the group.
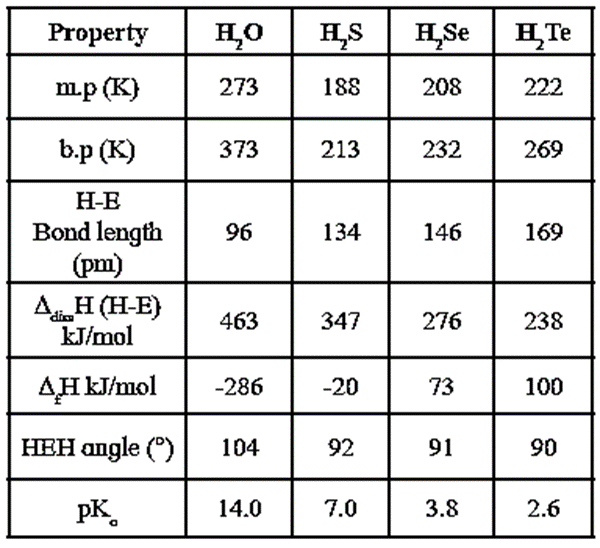
Physical states, volatility and chemical properties of hydrides of group 16 elements :
Physical state : Hydride of oxygen, H2O is a colourless odourless liquid while the hydrides of other group l6 elements are colourless poisonous gases with unpleasant odours.
Volatility : Volatility of hydrides increase from H2O to H2S and then decreases.
H2O < H2Te < H2Se < H2S
At ordinary temperature H20 is a liquid while all other hydrides are gases.
Thermal stability :
- The thermal stability of hydrides decreases in the order of H2O > H2S > H2Se > H2Te.
- Since atomic size increases from O to Te, the tendency to form hydride bond, M—H decreases. Hence M—H bond in O—H is the strongest and in Te—H the weakest. Therefore thermal stability decreases from H2O to H2Te.
Acidic character :
- The hydrides of group 16 elements are weakly acidic and acidic strength increases from H2O to H2Te.
- Since M—H bond strength decreases, bond enthalpy decreases, acidic character increases.
Reducing power :
- Except H2O, all hydrides of group 16 elements are reducing agents.
- Reducing power increases from H2S to H2Te.
- Reducing power of the hydrides is due to their less stability and tendency to dissociate, which increases from H2O to H2Te.
Q. Among the hydrides of group 16, water shows unusual properties. Why ?
Ans. Oxygen being more electronegative, the O-H bond is more polar and there arises association of H2O molecules through intermolecular hydrogen bonding.
The other hydrides of group 16 do not form H bonds and hence exist as discrete molecules.
As a result, water shows unusual properties like high B.P, high thermal stability and weaker acidic character as compared to the other hydrides of group 16.
(ii) Group 17 elements :
The elements of group 17 react with hydrogen to give hydrogen halides.
H2 + X2 → 2HX
(Where X = F, Cl, Br, I) Some of the properties of hydrogen halides are given in Table.
The affinity and reactivity decrease down the group from F to I.
H2 + F2 \(\underrightarrow{Dark}\) 2HF diffused
H2 + CL2 \(\underrightarrow{Diffused sunlight}\) 2HCl
H2 +Br2 \(\underrightarrow{Heat}\) 2HBr
H2 + I2 \(\underrightarrow{Heat}\) 2HI
Table : Properties of hydrides of group 17 elements.

Acidic strength :
- The acidic strength of haloacids vary in the following order, HF < HCl < HBr < HI
- The stability of hydrogen halides decreases down the group. HF > HCl > HBr > HI. This is because from F to I, atomic size increases and bond dissociation enthalpy of H—X decreases.
Thermal stability :
- HF is the most stable among all the hydrogen halides.
- The thermal stability decreases from HF to HI.
- This is due to decrease in bond dissociation enthalpy and bond strength of H—X bond from HF to HI.
- Down the group, from F to I, atomic size increases, bond length of H—X bond increases, bond polarity decreases and hence bond strength decreases.
- F being of the lowest atomic size and the highest electronegativity, HF bond is stronger and highly thermally stable.
Reducing character :
- The reducing character of hydrogen halides increases from HF to HI.
- HF does not show any reducing property while HI is a strong reducing agent.
- H—X bond strength and thermal stability of hydrogen halides decreases from HF to HI.
- Unlike other hydrogen halides, HF does not dissociate releasing hydrogen, hence HF is not a reducing agent.
(iii) Group 18 elements (Noble gases) :
- Noble gases are chemically inert towards hydrogen due to their stable electronic configuration.
Reactivity towards oxygen :
(i) Group 16 elements :
- All the elements of group 16 form oxides of the type EO2 and EO3 where E = S, Se, Te, Po.
- EO2 type oxides, Ozone (O3) and sulfur dioxide (SO2) are gases, while selenium dioxide (SeO2) is solid. They are acidic in nature and react with water to form acids.
SO2 + H2O → H2SO3 (Sulfurous acid)
SeO2 + H2O → H2SeO3 (Selenious acid)
- Reducing property of dioxides decreases from SO2 to TeO2.
- SO2 is reducing while TeO2 serves as an oxidising agent.
- EO3 type oxides, SO3, SeO3, TeO3 are also acidic in nature. They dissolve in water to form acids.
SeO3 + H2O → H2SeO4 (Selenic acid)
TeO3 + 3H2O → H6TeO6 (Telluric acid)
(ii) Group 17 elements :
- Elements of group 17 (Halogens) form many oxides with oxygen with different oxidation states of halogens, but most of them are unstable.
- Fluorine forms O2F2 and OF2.
- Chlorine forms oxides like Cl2O, Cl2O3 , Cl2O5, ClO3 , Cl2O7 . All oxides are oxidising agents.
- Bromine forms oxides like Br2O, BrO2, BrO3 and iodine forms I2O4, I2O5, I2O7.
(iii) Group 18 elements :
- Noble gas elements are chemically inert and do not directly react with oxygen.
Properties of oxides of halogens :
- Both O2F2 and OF2 are strong fluorinating agents.
- Only OF2 is thermally stable at 298 K. OF2 oxidises plutonium to PuF6. This reaction is used to remove Pu as PuF6 from spent nuclear fuel.
- The chlorine oxides Cl2O, ClO2, Cl2O6 and Cl2O7 are highly reactive oxidizing agents and tend to explode. ClO2 is used as a bleaching agent in the paper industry and textiles and in water treatment.
- Bromine forms oxides like Br2O, BrO2, BrO3, which are the least stable halogen oxides. They are powerful oxidising agents.
- Iodine forms oxides like I2O4, I2O5 and I2O2.
- These solids are insoluble in water and decompose
- on heating. I2O5 is a powerful oxidising agent and is used to determine the amount of carbon monoxide.
- For a particular halogen, higher oxides are more stable than the lower ones.
Reactivity towards halogens :
(i) Group 16 elements :
- The group 16 elements with halogens form a large number of halides of the type EX2, EX4 and EX6 where E is an element of the group and X is a halogen.
- The stability of the halides decreases in the order fluoride > chloride > bromide > iodide.
- Hexahalides, SF6, SeF6 and TeF6 are formed by direct combination. They are colourless gases.
- They have sp3d2 hybridisation and possess octahedral structure. SF6 is exceptionally stable halide for steric reasons.
- Tetrahalides, SF4, SeF4, TeF4, TeCl4 have sp3 hybridisation and thus trigonal bipyramidal geometry with one equatorial position occupied by a lone pair.
- Dihalides, SCl2, SeCl2, TeCl2 have sp3 hybridisation and thus possess tetrahedral structure with two equatorial positions occupied by lone pairs.
- Monohalides are dimeric in nature. For example, S2F2, S2Cl2, Se2Cl2 and SeBr2. These dimeric halides undergo disproportionation.
2Se2Cl2 → SeCl4 + 3Se
(ii) Group 17 elements :
- The halogens have a tendency to combine amongst themselves forming different compounds called interhalogen compounds.
- Interhalogens are of the type XX, XX'3, XX’5 and XX’7 where X is larger size halogen than X’.
- They are covalent, diamagnetic, reactive and good oxidising agents.
- Preparation :
Cl2 + F2 \(\underrightarrow{473\,K}\) 2ClF
Br2 + 3P2 \(\underrightarrow{Δ}\) 2BrF3
(iii) Group 18 elements :
- Group 18 elements are chemically inert.
- However, inert elements like krypton and xenon react directly with fluorine under appropriate conditions to give their fluorides.
For example,
Xe(g) + F2 (g) \(\underrightarrow{673\,K\, 1atm}\) XeF2(s)
- The xenon fluorides XeF2, XeF4 and XeF6 are colourless crystalline solids which sublime at 298 K.
- These fluorides are strong fluorinating agents.
Reactivity towards metals :
(i) Group 16 elements :
Elements of group 16 react with metals to form corresponding compounds.
e.g.
4Al + 3O2 → 2Al2O3 (Aluminium oxide)
Cu + S → CuS (Copper sulphide)
Mg + Se → MgSe (Magnesium selenide)
(ii) Group 17 elements :
- Elements of group 17 (Halogens) react with metals instantly to give metal halides.
2Na(s) + Cl2(l) → 2NaCl(s)
Mg(s) + Br2(l) → MgBr2(s) magnesium bromide
- Ionic character of halides decreases in the order MF > MCl > MBr > MI, where M is a monovalent metal.
- The metal halides having metals in their higher oxidation states are more covalent than the ones having metals in lower oxidation state.
- For example, SnCl4, PbCl4, SbCl5 and UF6 are more covalent than SnCl2, PbCl2, SbCl3 and UF4 respectively.
(iii) Group 18 elements : Noble gases do not directly react with metals.
Chapter-7-Elements of Groups 16, 17, and 18-Text Book-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Notes-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Solution-PDF
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6-Chemical Kinetics – Online Notes
Next Chapter : Chapter-8-Transition and Inner transition Elements – Online Notes
We reply to valid query.