Structure of Atom
Class-11-Science-Chemistry-Chapter -4 Maharashtra State Board
Solution
Question 1.
Choose correct option.
(A) The energy difference between the shells goes on ........... when moved away from the nucleus.
(a) Increasing
(b) decreasing
(c) equalizing
(d) static
(b) decreasing
(B) The value of Plank’s constant is -
(a) 6.626 × 10-34Js
(b) 6.023 × 10-24Js
(c) 1.667 × 10-28Js
(d) 6.626 × 10-28Js
(a) 6.626 × 10-34Js
(C) p-orbitals are....... in shape.
(a) spherical
(b) dumb bell
(c) double dumbell
(d) diagonal
(b) dumb bell
(D) “No two electrons in the same atoms can have identical set of four quantum numbers”. This statement is known as -
(a) Pauli’s exclusion principle
(b) Hund’s rule
(c) Aufbau rule
(d) Heisenberg uncertainty principle
(a) Pauli’s exclusion principle
(E) Principal Quantum number describes
(a) shape of orbital
(b) size of the orbital
(c) spin of electron
(d) orientation of in the orbital electron cloud
(b) size of the orbital
Question 2.
Make the pairs:
| ‘A’ | ‘B’ |
| a. Neutrons | i. six electrons |
| b. p-orbital | ii.-1.6 × 10-19 C |
| c. charge on electron | iii. Ultraviolet region |
| d. Lyman series | iv. Chadwick |
‘A’
‘B’
a. Neutrons
iv. Chadwick
b. p-orbital
i. six electrons
c. charge on electron
ii.-1.6 × 10-19 C
d. Lyman series
iii. Ultraviolet region
Question 3.
Complete the following information about the isotopes in the chart given below.
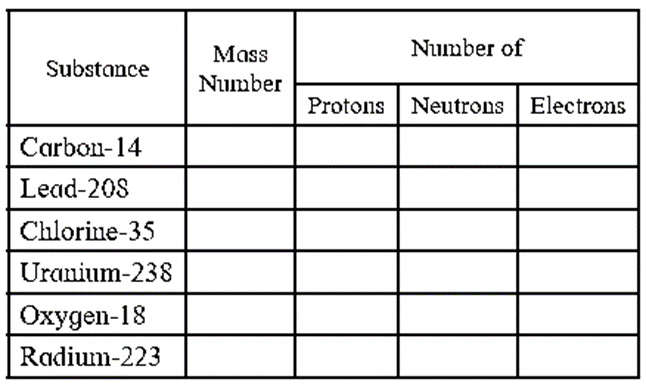
(Hint: Refer to Periodic Table if required)
(A) (Z) (A-Z)
Substance
Mass Number
Number of
Protons
Neutrons
Electrons
Carbon-14
14
6
8
6
Lead-208
208
82
126
82
Chlorine-35
35
17
18
17
Uranium-238
238
92
146
92
Oxygen-18
18
8
10
8
Radium-223
223
88
135
88
Question 4.
Match the following :
| Element | No. of Neutron |
| (a) \(_{18}^{40}\text{Ar}\) | (i) 7 |
| (b) \(_{6}^{14}\text{C}\) | (ii) 21 |
| (c) \(_{19}^{40}\text{K}\) | (iii) 8 |
| (d) \(_{7}^{14}\text{N}\) | (iv) 22 |
Element
No. of Neutron
(a) \(_{18}^{40}\text{Ar}\)
(iv) 22
(b) \(_{6}^{14}\text{C}\)
(iii) 8
(c) \(_{19}^{40}\text{K}\)
(ii) 21
(d) \(_{7}^{14}\text{N}\)
(i) 7
Question 5.
Answer in one sentence.
(A) If an element ‘X’ has mass number 11 and it has 6 neutrons, then write its representation.
Atomic number = 11 − 6 = 5 Element is \(_{5}^{11}\text{B}\)
(B) Name the element that shows simplest emission spectrum.
Since hydrogen atom has one electron it shows the simplest emission spectrum.
(C) State Heisenberg uncertainty principle.
Heisenberg uncertainty principle : This principle states that it is not possible to determine simultaneously the position and momentum of a moving microscopic particle like electron with absolute certainty.
(D) Give the names of quantum numbers.
Quantum numbers are as follows :
(E) Identify from the following the isoelectronic species:
Ne, O2−, Na+ OR Ar, Cl−, K+
∴Isoelectronic species : (i) Ne O2− Na+ (ii) Ar Cl− K+
Species
Ne O2− Na+
Ar Cl− K+
Number of electrons
10 10 10
18 18 18
Question 6.
Answer the following questions.
(A) Differentiate between Isotopes and Isobars.
Isotopes
Isobars
Isotopes are the atoms of the same element.
Isobars are the atoms of different elements.
Isotopes have same atomic number but different mass numbers.
Isobars have different atomic numbers but same mass number.
They have same number of electrons.
They have different number of electrons.
They have same number of protons but different number of neutrons.
They have different number of protons and neutrons.
They have same chemical properties.
They have different chemical properties.
For Example \(_{17}^{35}\text{Cl}\) and \(_{17}^{34}\text{Cl}\)
For example \(_{18}^{40}\text{Ar}\), \(_{19}^{40}\text{K}\), \(_{20}^{40}\text{Ca}\)
(B) Define the terms:
(i) Isotones
Isotones : The atoms of different elements having same number of neutrons in their nuclei are called isotones.
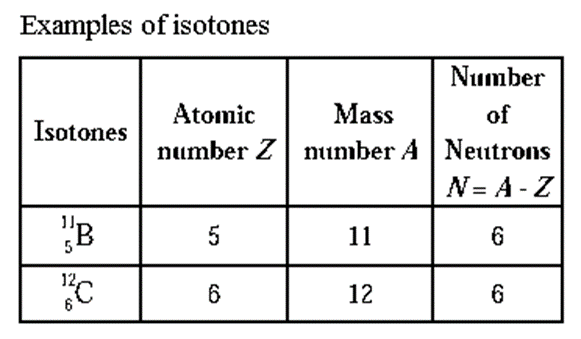
(ii) Isoelectronic species.
Isoelectronic species : Atoms and ions having the same number of electrons are isoelectronic species. Example : Consider K+ formed by removal of one electron from K atom. Which has 19 electrons (Z = 19). Therefore K+ has 18 electrons. Species such as Ar, Ca2+ containing 18 electrons are isoelectronic with K+ . Electronic configuration of all these species with 18 electrons is 1s2, 2s2, 2p6, 3s2, 3p6.
Species
K→ K+ + e−
Ar, Ca2+
Number of electrons
19 18
18 18
(iii) Electronic configuration.
Electronic configuration of an atom is the distribution of its electrons in orbitals. The electronic configuration can be written by applying the aufbau principle. There are two methods of representing electronic configuration: (a) Orbital notation: nsa npb ndc.......... For example for boron atom 5B 1s2, 2s2, 2p1. (b) Orbital diagram:
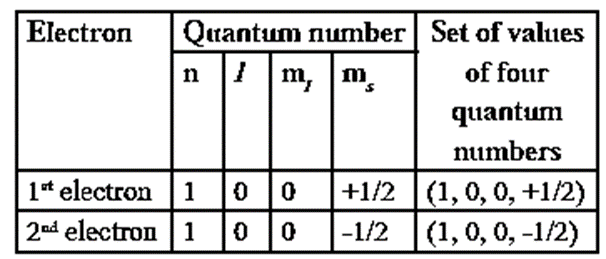
(C) State and explain Pauli’s exclusion principle.
The capacity of an orbital to accommodate electrons is decided by Pauli exclusion principle. Statement of Pauli’s principle : No two electrons in an atom can have all the four quantum numbers, (n, l, m and s) same. OR Only two electrons may exist in the given orbital having three quantum numbers same but fourth quantum number being different with opposite spins. This principle describes the capacity of a sub-shell or orbital to accommodate maximum number of electrons. Consider helium atom, which has two electrons. The four quantum numbers of two electrons in He atom will be, Hence 1s orbital can accommodate two electrons. This principle is illustrated with helium atom He (Z = 2). Its electronic configuration is 1s2 as
(D) State Hund’s rule of maximum multiplicity with suitable example.
Statement of Hund’s rule of maximum multiplicty : It states that pairing of electrons in the orbitals belonging to the same subshell does not occur unless each orbital belonging to that subshell has accommodated one electron each. After filling three electrons, one in each with same spins, the next electrons enter with pairing It observed that half filled and completely filled set of degenerate orbitals have extra stability.

(E) Write the drawbacks of Rutherford’s model of an atom.
Rutherford’s atomic model has following drawbacks :
(F) Write postulates of Bohr’s Theory of hydrogen atom.
Postulates of Bohr atomic theory Bohr’s model of hydrogen atom is based on the following postulates. (i) The electron in the hydrogen atom can move around the nucleus in certain permitted circular orbits arranged concentrically in increasing order of energy. (ii) The energy of an electron in the orbit does not change with time. Hence orbits are called stationary orbits. On absorption of required energy electron moves from lower orbit to higher orbit. The transition from higher energy orbit to lower energy orbit is accompanied by emission of energy in the form of electromagnetic radiation. (iii) The frequency ν of radiation absorbed or emitted on transition between two stationary orbits differing by energy ΔE is given by, ν = ΔE/h = E2 − E1 where E1 and E2 are the energies of the lower and higher energy states respectively. Above equation represents Bohr’s frequency rule. (iv) An electron can occupy only those orbits in which its angular momentum is integral (n) multiple of h/2π Angular momentum = \(\frac{nh}{2π}\)
(G) Mention demerits of Bohr’s Atomic model.
Demerits of Bohr model
(H) State the order of filling atomic orbitals following Aufbau principle.
Aufbau is a German word which means building up. This principle explains the sequence of filling up of orbitals with electrons. Aufbau principle : It states that in the ground state of an atom, the orbitals are filled with electrons in order of the increasing energies. The orbitals are filled in order of increasing value of (n + l). For example, 4s-orbital (n + l = 4 + 0 = 4) is filled prior to 3d-orbital (n + l = 3 + 2 = 5). Among two orbitals having same (n + l) value, that orbital with lower value of n will be filled first. For example, 3d-orbital (n + l = 3 + 2 = 5) is filled prior to 4p-orbital (n + l = 4 + 1 = 5). The increasing order of energy of different orbitals is as follows : 1s<2s<2p<3s<3p<4sz<3d<4p<5s<4d<5p<6s<4f<5d<6p<7s<5f<6d

(I) Explain the anomalous behavior of copper and chromium.
Anomalous behaviour of copper : Copper (29Cu) has electronic configuration. 29Cu (Expected) : 1s2, 2s2, 2p6, 3s2, 3p6, 3d9, 4s2 (Observed) : 1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s1 Explanation : Anomalous behaviour of chromium : Chromium (24Cr) has electronic configuration, 24Cr (Expected) : 1s2, 2s2, 2p6, 3s2, 3p6, 3d4, 4s2 (Observed) : 1s2, 2s2, 2p6, 3s2, 3p6, 3s2, 4s1 Explanation :
(J) Write orbital notations for electrons in orbitals with the following quantum numbers.
(a) n = 2, l =1 (b) n = 4, l = 2 (c) n = 3, l = 2
(a) n = 2, l = 1 the orbital is 2p (b) n = 4, l = 2 the orbital is 4d (c) n = 3, l = 2, the orbital is 3d
(K) Write electronic configurations of Fe, Fe2+, Fe3+
26Fe [Ar] 3d6 4s2 Fe2+ [Ar] 3d6 Fe3+ [Ar] 3d5
(L) Write condensed orbital notation of electronic configuration of the following elements:
(a) Lithium (Z=3) (b) Carbon (Z=6)
(c) Oxygen (Z=8) (d) Silicon (Z=14)
(e) Chlorine (Z=17) (f) Calcium (Z=20)
(a) 3Li [He] 2s1 (b) 6C [He] 2s2 2p2 (c) 8O [He] 2s2 2p4 (d) 14Si [Ne] 3s2 3p2 (8) 17Cl [Ne] 3s2 3p5 (f) 20Ca [Ar] 4s2
(M) Draw shapes of 2s and 2p orbitals.
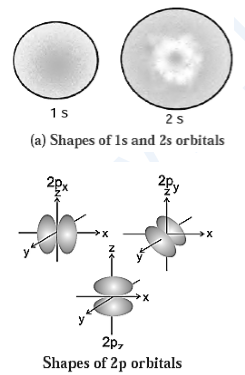
(N) Explain in brief, the significance of azimuthal quantum number.
Azimuthal quantum number (l) : This represents the subsidiary quantum number or the subshell of the orbit to which the electron belongs. It is denoted by l. It is also called as secondary, subsidiary, orbital or angular momentum quantum number. It defines the shape of the orbital and the angular momentum of the electron. The value of l depends on principal quantum number n. It has positive values between 0 to (n — 1) : This explains the significance of azimuthal quantum number
l
0
1
2
3
subshell
s
p
d
f
(O) If n=3, what are the quantum number l and m?
For n = 3, l = 0, 1, 2. For l = 0, ml = 0 For l = 1, ml = −1, 0, +1 For l = 2, ml = −2, −1, 0, +1
(P) The electronic configuration of oxygen is written as 1s2 2s2 2px2 2py1 2pz1 and not as 1s2 2s2 2px2 2py2 2pz0, Explain.
By Hund's rule, 1s2 2s2 2px2 2py1 The electronic configuration, 1s2 2s2 2px2 2py2 2pz0 violates Hund's rule
(Q) Write note on ‘Principal Quantum number.
Principal quantum number (n) : This describes the orbit or shell of an atom to which the electron belongs. It is represented by ‘n’ which has integral values. The energy of an electron depends on the value of n. ↓
Principal quantum number n
Shell symbol
Allowed number of orbitals n2
Size of shell
1
K
1
Increases
2
L
4
3
M
9
4
N
16
(R) Using concept of quantum numbers, calculate the maximum numbers of electrons present in the ‘M’ shell. Give their distribution in shells, subshells and orbitals.
‘M’ shell has, n = 3 l = 0, 1, 2 ml : When l = 0, ml = 0, ms = \(±\frac{1}{2}\) ml : When l = 1, ml = −1, 0, +1 ms = \(±\frac{1}{2}\),\(±\frac{1}{2}\),\(±\frac{1}{2}\) ml : When l = 2, ml = −2, −1, 0, +1, +2 ms = \(±\frac{1}{2}\),\(±\frac{1}{2}\),\(±\frac{1}{2}\),\(±\frac{1}{2}\),\(±\frac{1}{2}\)
(S) Indicate the number of unpaired electrons in :
(a) Si (Z=14) (b) Cr (Z=24)
(a) 14Si [Ne] 3s2 3px1; 3py1; Number of unpaired electrons = 2 (b) 24Cr [Ar] 3d5 4s1 Number of unpaired electrons = 6
(T) An atom of an element contains 29 electrons and 35 neutrons. Deduce
(a) the number of protons
(b) the electronic configuration of that element
(a) Since atom has 29 electrons, it has 29 protons. The atomic number of the element is 29. It is copper. (b) ∴ Electronic configuration : 29Cu [Ar] 3d10 4s1.
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-3-Basic Analytical Techniques – Online Solutions
Next Chapter : Chapter-5-Chemical Bonding – Online Solutions
We reply to valid query.