Chemical Thermodynamics
Maharashtra Board-Class-12-Chemistry-Chapter-4
Solutions
Question 1. Select the most apropriate option.
(i) The correct thermodynamic conditions for the spontaneous reaction at all temperatures are
(a) ΔH < 0 and ΔS > 0
(b) ΔH > 0 and ΔS < 0
(c) ΔH < 0 and ΔS < 0
(d) ΔH < 0 and ΔS = 0
(a) ΔH < 0 and ΔS > 0
(ii) A gas is allowed to expand in a well insulated container against a constant external pressure of 2.5 bar from an initial volume of 2.5 L to a final volume of 4.5 L. The change in internal energy, ΔU of the gas will be
(a) —500 J
(b) + 500 J
(c) —1013 J
(d) + 1013 J
(a) —500 J
(iii) In which of the following, entropy of the system decreases?
(a) Crystallization of liquid into solid
(b) Temperature of crystalline solid is increased from 0 K to 115 K
(c) H2(g) → 2H(g)
(d) 2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
(a) Crystallization of liquid into solid
(iv) The enthalpy of formation for all elements in their standard states is
(a) unity
(b) zero
(c) less than zero
(d) different elements
(b) zero
(v) Which of the following reactions is exothermic?
(a) H2(g) → 2H(g)
(b) C(s) → C(g)
(c) 2Cl(g) → Cl2(g)
(d) H2O(s) → H2O(l)
(c) 2Cl(g) → Cl2(g)
(vi) 6.24 g of ethanol are vaporized by supplying 5.89 kJ of heat. Enthalpy of vaporization of ethanol will be
(a) 43.4 kJ mol—1
(b) 60.2 kJ mol—1
(c) 38.9 kJ mol—1
(d) 20.4 kJ mol—1
(a) 43.4 kJ mol—1
(vii) If the standard enthalpy of formation of methanol is —238.9 kJ mol—1 then entropy change of the surroundings will be
(a) —801.7 J K—1
(b) 801.7 J K—1
(c) 0.8017 J K—1
(d) —0.8017 J K—1
(b) 801.7 J K—1
(viii) Which of the following are not state functions?
(1) Q + W (2) Q (3) W (4) H—TS
(a) 1,2 and 3
(b) 2 and 3
(c) 1 and 4
(d) 2,3 and 4
(b) 2 and 3
(ix) For vaporization of water at 1 bar, ΔH = 40.63 kJ mol—1 and ΔS = 108.8 JK—1 mol—1. At what temperature, ΔG = 0 ?
(a) 273.4 K
(b) 393.4 K
(c) 373.4 K
(d) 293.4 K
(c) 373.4 K
(x) Bond enthalpies of H—H, Cl—Cl and H—Cl bonds are 434 kJ mol—1, 242 kJ mol—1 and 431 kJ mol—1, respectively. Enthalpy of formation of HCl is
(a) 245 kJ mol—1
(b) —93 kJ mol—1
(c) —245 kJ mol—1
(d) 93 kJ mol—1
(c) 373.4 K
Question 2. Answer the following in one or two sentences.
(i) Comment on the statement: no work is involved in an expansion of gas in vacuum.
- When a gas expands against an external pressure Pex, changing the volume from V1 to V2, the work obtained is given by
W = −Pex (V2 − V1).
- Hence the work is performed by the system when it experiences the opposing force or pressure.
- Greater the opposing force, more is the work.
- In free expansion, the gas expands in vacuum (where it does not experience opposing force, (P = 0). Since external pressure is zero, no work is obtained.
W = −Pex (V2 − V1)
= −0 x (V2 − V1) = 0
- Since during expansion in vacuum no energy is expended, it is called free expansion.
(ii) State the first law of thermodynamics.
According to this law the total energy of a system and surroundings remains constant when the system changes from an initial state to final state.
The law is stated in different ways as follows :
- Energy can neither be created nor destroyed, however, it may be converted from one form into another.
- Whenever, a quantity of one kind of energy is consumed or disappears, an equivalent amount of another kind of energy appears.
- The total mass and energy of an isolated system remain constant, although there may be inter—conservation of energy from one form to another.
- The total energy of the universe remains constant.
(iii) What is enthalpy of fusion?
Enthalpy of fusion (ΔfusH) : The enthalpy change that accompanies the fusion of one mole of a solid into a liquid at constant temperature and pressure is called enthalpy of fusion.
For example,
H2O(s) H2O(l), \(\underrightarrow{1\,atm\,273\,K}\) ΔfusH = 6.01 kJ mol−1
This equation describes that when one mole of ice melts (fuses) at 0°C (273 K) and 1 atmosphere, 6.01 kJ of heat will be absorbed.
(iv) What is standard state of a substance?
The thermodynamic standard state of a substance (compound) is the most stable physical state of it at 298 K and 1 atmosphere (or 1 bar). The enthalpy of the substance in the standard state is represented as ΔfH0.
(v) State whether ΔS is positive, negative or zero for the reaction
2H(g) → H2(g). Explain.
The given reaction, 2H(g) → H2(g) is the formation of H2(g) from free atoms.
Since two H atoms form one H2 molecule, Δn = 1 — 2 = — 1 and disorder is decreased. Hence entropy change ΔS < 0 (or negative).
(vi) State second law of thermodynamics in terms of entropy.
Second law of thermodynamics in terms of entropy :
The second law of thermodynamics states that the total entropy of the system and its surroundings (universe) increases in a spontaneous process.
It is expressed mathematically as
ΔStotal = ΔSsystem + ΔSsurr > 0 (for spontaneous process)
ΔSuniverse = ΔSsystem + ΔSsurr > 0
The total entropy increases during a spontaneous process that finally reaches equilibrium.
(vii) If the enthalpy change of a reaction is ΔH how will you calculate entropy of surroundings?
(a) For endothermic reaction, ΔH > 0. This shows the system absorbs heat from surroundings.
∴ Δsurr H < 0.
∴ Entropy change = Δsurr S = \(\frac{Δ_{surr}\,H}{T}\)
There is decrease in entropy of surroundings.
(b) For exothermic reaction, ΔH < 0, hence for surroundings, Δsurr H > 0
∴ Δsurr > 0.
(viii) Comment on spontaneity of reactions for which ΔH is positive and ΔS is negative.
Spontaneity of reactions when ΔH is positive and ΔS is negative: If ΔH is positive, (ΔH > 0) and ΔS is negative (ΔS < 0), ΔG will be always positive (ΔG > 0) and hence the reaction will be non—spontaneous at all temperatures—
Question 3. Answer in brief.
(i) Obtain the relationship between ΔG0 of a reaction and the equilibrium constant.
Consider following reversible reaction,
aA + bB ⇌ cC + dD
The reaction quotient Q is,
Q = \(\frac{[C]^c×[D]^d}{[A]^a×[B]^b}\)
The free energy change ΔG for the reaction is
ΔG = ΔG° + RT ln Q
Where ΔG° is the standard free energy change.
At equilibrium
Q = \(\frac{[C]^e_c×[D]^e_d}{[A]^e_a×[B]^e_b}\) = K
ΔG = ΔG° + RT ln K
at equilibrium ΔG = 0
0 = ΔG° + RT ln K
ΔG° = —RT ln K
ΔG° = — 2.303 RT log10K.
(ii) What is entropy? Give its units.
(a) Entropy : Being a state function and thermodynamic function it is defined as entropy change (ΔS) of a system in a process which is equal to the amount of heat transferred in a reversible manner (Qrev) divided by the absolute temperature (T), at which the heat is absorbed. Thus,
Entropy change = \(\frac{\text{Heat transferred reversibly}}{\text{Absolute temperature of transfer}}\)
∴ ΔS = Qrev/T
Entropy is a measure of disorder in the system Higher the disorder, more is entropy of the system
(b) SI units of entropy are JK−1 and c.g.s. units are cal K−1. It is also expressed in terms of entropy unit (e.u.). Hence 1 e.u. = 1 J K−1.
(iii) How will you calculate reaction enthalpy from data on bond enthalpies?
Reaction and bond enthalpies :
- In a chemical reaction bonds are broken and formed.
- The enthalpies of reactions involving substances having covalent bonds are calculated by knowing the bond enthalpies of reactants and those in products.
- The calculations assume all the bonds of a given type are identical.
- Energy is always required to break a chemical bond while energy is always released in the formation of the bond.
The enthalpy change of a gaseous reactions (ΔrH°) involving substances with covalent bonds can be calculated with the help of bond enthalpies of reactants and products. (In case of solids we need lattice energy or heat of sublimation while in case of liquids we need heat of evaporation.)
ΔH°(reaction) = [Sum of bond enthalpies of bonds broken of reactants] — [Sum of bond enthalpies of bonds formed of products]
= ∑ ΔH°(bonds broken) − ∑ ΔH°(bonds formes)
For example for a following reaction,
H2(g) + Cl2(g) → 2HCl(g) OR
H—H(g) + Cl—Cl(g) → 2H—Cl(g)
ΔH°(reaction) = [ΔH°H—H + ΔH°Cl—Cl] — 2[ΔH°H—Cl]
If the energy required to break the bonds of reacting molecules is more than the energy released in the bond formation of the products, then the reaction will be endothermic and ΔH° reaction will be positive.
On the other hand if the energy released in the bond formation of the products is more than the energy required to break the bonds of reacting molecules then the reaction will be exothermic and ΔH° reaction will be negative.
(iv) What is the standard enthalpy of combustion ? Give an example.
Standard enthalpy of combustion or standard heat of combustion : It is defined as the enthalpy change when one mole of a substance in the standard state undergoes complete combustion in a sufficient amount of oxygen at constant temperature (298 K) and pressure (1 atmosphere or 1 bar). It is denoted by ΔCH°. p
E.g. C2H2(g)+ \(\frac{5}{2}\)O2(g) → 2CO2(g)+ H2O(l),
ΔCH0 = −1300 kJ mol−1, (ΔCH0 is always negative.)
In the above reaction, the standard enthalpy change of the oxidation reaction, −1300 kJ is the standard enthalpy of combustion of C2H2(g).
(v) What is the enthalpy of atomization? Give an example.
Enthalpy of atomisation (ΔatoH) : The enthalpy change or amount of heat absorbed accompanying the dissociation of the molecules in one mole of a gaseous substance into free gaseous atoms at constant temperature and pressure is called enthalpy of atomization.
For example,
(a) Cl2(g) → 2Cl(g) ΔatoH = 242 kJ mol−1
(b) CH4(g) → C(g) + 4H(g) ΔatoH = 1660 kJ mol−1
(vi) Obtain the expression for work done in chemical reaction.
Consider n1 moles of gaseous reactants A of volume V1 change to n2 moles of gaseous products B of volume V2 at temperature T and pressure P.
In the initial state, PV1 = n1RT
In the final state, PV2 = n2RT
PV2 − PV1 = n2RT— n1RT = (n2 − n1)RT = ΔnRT
where Δn is the change in number of moles of gaseous products and gaseous reactants.
Due to net changes in gaseous moles, there arises change in volume against constant pressure resulting in mechanical work, — PΔV.
W = −PΔV= −P (V2 − V1) = −ΔnRT
Meaning of each term :
- If n1 = n2, Δn = 0, W = 0. No work is performed.
- If n2 > n1, Δn > 0, there is a work of expansion by the system and W is negative.
- If n2 < n1, Δn< O, there is a work of compression, hence work is done on the system and W is positive.
(vii) Derive the expression for PV work.
Consider a certain amount of gas at constant pressure P is enclosed in a cylinder fitted with frictionless, rigid movable piston of area A. Let volume of the gas be V1 at temperature T.

As the gas expands, it pushes the piston upward through a distance d against external force F pushing the surroundings.
The work done by the gas is,
W = opposing force x distance
= − F x d
− ve sign indicates the lowering of energy of the system during expansion.
If a is the cross section area of the cylinder or piston, then,
W = \(-\frac{F}{a}\) x d x a
Now the pressure IS Pex = F/a
while volume change is, ΔV = d x a
∴ W = −Pex x ΔV
If during the expansion, the volume changes from V1 and V2 then, ΔV = V2 − V1
∴ W = − Pex(V2 − V1)
During compression, the work W is + ve, since the energy of the system is increased,
W = +Pex(V2 − V1)
(viii) What are intensive properties? Explain why density is intensive property.
(a) Intensive property : A property which is independent of the amount of matter in a system is called intensive property.
- Intensive property is characteristic of the system. E.g. density, viscosity, pressure, temperature etc.
- The intensive properties are not additive
(b) Density is a ratio of two extensive properties namely, mass and volume. Since the ratio of two extensive properties represents an intensive property, density is an intensive property. It does not depend on the amount of a substance.
(ix) How much heat is evolved when 12 g of CO reacts with NO2 ? The reaction is :
4CO(g) + 2 NO2(g) → 4CO2(g) + N2(g),
ΔrH0 = −1200 kJ
Given : 4CO(g) + 2NO2(g) → 4CO2(g) + N2(g)
Molar mass of CO = 28 g mol—1, ΔrH0 = − 1200 kJ,
mCO = 12 g, ΔH = ?
From the reaction,
For 4 x 28g CO ΔH°= − l200 kJ
For 12 g CO ΔH° = \(\frac{-1200×12}{4×28}\) = − 128.6 kJ
Answer is : Heat evolved = 128.6 kJ.
Question 4. Answer the following questions.
(i) Derive the expression for the maximum work.
Consider n moles of an ideal gas enclosed in an ideal cylinder fitted with a massless and frictionless movable rigid piston.
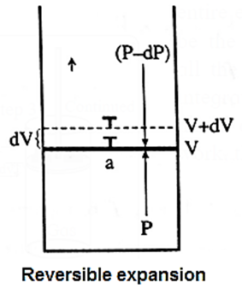
Let V be the volume of the gas at a pressure P and a temperature T.
If in an infinitesimal change pressure changes from P to P — dP and volume increases from V to V + dV. Then the work obtained is,
dW = − (P − dP)dV
= − PdV + dPdV
Since dP.dV is negligibly small relative to PdV
dW = − PdV
Let the state of the system change from A(P1, V1) to B (P2, V2) isothermally and reversibly, at temperature T involving number of infinitesimal steps.
A(P1, V1) ------T------> B (P2, V2)
Then the total work or maximum work in the process is obtained by integrating above equation.
Wmax = \(\int_{V_1}^{V_2} dW = \int_A^B -pdv\)
‘.’ PV = nRT
∴ P = nRT/V
Wmax = \(\int_A^B -nRT\frac{dV}{V}\)
= \(-nRT\int_A^B \frac{dV}{V}\)
= −nRT(lnV2 − lnV1)
= −nRT loge\(\frac{V_2}{V_1}\)
∴ Wmax = −2.303 nRT log10 \(\frac{V_2}{V_1}\)
At constant temperature,
‘.’ P1 x V1 = P2 x V2
\(\frac{V_2}{V_1}\) = \(\frac{P_1}{P_2}\)
Wmax = −2.303 nRT log10 \(\frac{P_1}{P_2}\)
where n, P, V and T represent number of moles, pressure, volume and temperature respectively.
For the process,
Δ U = 0, Δ H = 0.
Note : The heat absorbed in reversible manner Qrev, is completely convened into work Qrev = Wmax Hence work obtained is maximum.
(ii) Obtain the relationship between ΔH and ΔU for gas phase reactions.
Consider a reaction in which n1 moles of gaseous reactant in initial state change to n2 moles of gaseous product in the final state.
Let H1, U1, P1, V1 and H2, U2, P2, V2 represent enthalpies, internal energies, pressures and volumes in the initial and final states respectively then,
n1A(g) → n2B(g)
The heat of reaction is given by enthalpy change ΔH as,
ΔH = H2 — H1
By definition, H = U + PV
∴ H1 = U1 + P1V1 and H2 = U2 + P2V2
∴ ΔH = (U2 + P2V2) — (U1 + P1V1)
= (U2 — U1) + (P2V2 — P1V1)
Now, ΔU= U2 — U1
Since PV= nRT,
For initial state, P1V1= n1RT
For final state, P2V2= n2RT
∴ P2V2 — P1V1= n2RT— n1RT
= (n2 — n1) RT = ΔnRT
Where Δn = [ Number of moles of gaseous products] — [Number of moles of gaseous reactants]
ΔH = ΔU + ΔnRT
If QP, and QV, are the heats involved in the reaction at constant pressure and constant volume respectively, then since QP = ΔH and QV = ΔU.
∴ QP = QV + ΔnRT
(iii) State Hess’s law of constant heat summation. Illustrate with an example. State its applications.
The law states that, “Overall the enthalpy change for a reaction is equal to sum of enthalpy changes of individual steps in the reaction”. OR
Heat of reaction is same whether it is carried out in one step or in several steps.
Explanation :
Consider the formation of CO2(g). .
In one step : C(g) + O2(g) → CO2(g) ΔH = — 394 kJ
In two steps : C(s) + 1/2 O2(g) → CO(g) ΔH1 = —83 kJ
CO(g) + 1/2 O2(g) → CO2(g) ΔH2 = —311 kJ
ΔH = ΔH1+ ΔH2
—394 kJ = —83kJ + (—311) kJ
Hess’s law treats thermochemical equations mathematically i.e., they can be added, subtracted or multiplied by numerical factors like algebraic equations.
Applications of Hess’s law :
The Hess's law has been useful to calculate the enthalpy changes for the reactions with their enthalpies being not known experimentally.
It is used,
- To calculate heat of formation, combustion, neutralisation, ionization, etc.
- To calculate the heat of reactions which may not take place normally or directly.
- To calculate heats of extremely slow or fast reactions.
- To calculate enthalpies of reactants and products.
(iv) Although ΔS for the formation of two moles of water from H2 and O2 is —327 JK—1, it is spontaneous. Explain. (Given ΔH for the reaction is —572 kJ).
Given = ΔS: — 327 JK—1; ΔH = — 572 kJ
‘.’ ΔG = ΔH — TΔS, and ΔH << ΔS
∴ ΔG < 0, and hence the formation of H2O(l) is spontaneous.
(v) Obtain the relation between ΔG and ΔS total Comment on spontaneity of the reaction.
Gibbs free energy, G is defined as,
G = H — TS
where H is the enthalpy, S is the entropy of the system at absolute temperature T.
Since H, T and S are state functions, G is a state function and a thermodynamic function.
At constant temperature and pressure, change in free energy ΔG for the system is represented as,
ΔG = ΔH — T ΔS
Free energy change = Total enthalpy change —Temperature x Entropy change
This is called Gibbs free energy equation for ΔG.
In this ΔS is total entropy change, i.e., ΔSTotal.
The SI units of ΔG are J or kJ (or J mol−1 or kJ mol−1).
The c.g.s. units of ΔG are cal or kcal (or cal mol−1 or kcal mol−1)
The second law explains the conditions of spontaneity as below :
- ΔStotal > o and ΔG < 0, the process is spontaneous.
- ΔStotal < 0 and ΔG > 0, the process is non spontaneous.
- ΔStotal = 0 and ΔG = 0, the process is at equilibrium.
(vi) One mole of an ideal gas is compressed from 500 cm3 against a constant external pressure of 1.2 × 105 Pa. The work involved in the process is 36.0 J. Calculate the final volume.
Given : V1 = 500 cm3 = 0.5 dm3; Pext = 1.2 x105 Pa = 1.2 bar; W = 36 J;
1dm3bar = 100 J; V2 = ?
W = −Pext(V2 − V1)
36 J = − 1.2(V2 − 0.5) dm3 bar
= −1.2(V2 − 0.5) x 100 J
V2 − 0.5 = \(-\frac{36}{1.2×100}\) = −0.3
∴ V2 = 0.5 − 0.3 = 0.2 dm3 = 200 cm3
Answer is : Final volume = 200 cm3.
(vii) Calculate the maximum work when 24 g of O2 are expanded isothermally and reversibly from the pressure of 1.6 bar to 1 bar at 298 K.
Given : WO2 = 24 g, P1 = 1.6 bar, P2 = 1 bar, T= 298 K, Wmax = ?
Wmax = −2.303 nRT log10 \(\frac{P_1}{P_2}\)
= − 2.303 x \(\frac{W_{o2}}{M_{o2}}\) x 8.314 x 298 x log10 \(\frac{1.6}{1}\)
= − 2.303 x \(\frac{24}{32}\) x 8.314 x 298 x 0.2041
= − 873.4 J
Answer is Wmax = − 873.4 J.
(viii) Calculate the work done in the decomposition of 132 g of NH4NO3 at 100 0C. NH4NO3 (s) → N2O(g) + 2H2O(g) State whether work is done on the system or by the system.
Given :
NH4NO3 (s) → N2O(g) + 2H2O(g)
= 132 g;
Molar mass of NH4NO3 = (2 x 14) + (3 x 16) + (4 x 1) = 80 g mol—1
= 80 g mol—1
T = 273 + 100 = 373K; Δn = ?
For the reaction,
Δn = ∑n2 gaseous products − ∑n1 gaseous products = 3 − 0 = 3
Since there is an increase in number of gaseous moles, the work is done by the system.
Moles of NH4NO3 = n = \(\frac{m_{NH_4NO_3}}{M_{NH_4NO_3}}\) = \(\frac{132}{80}\) = 1.65 mol
The given reaction is for 1 mole of NH4NO3 Δn = 3 mol
For 1.65 moles of NH4NO3 the reaction is
1.65 NH4NO3 → 1.65 N2O + (2 x 1.65) H2O
∴ 1.65 NH4NO3 → 1.65 N2O + (3.30) H2O
Δn = (moles of gaseous products) – (moles of gaseous reactants)
= (1.65 + 3.30) – 0 = 4.95 mole …..(‘.’ NH4NO3 is in solid state)
W = − ΔnRT = −4.95 x 8.314 x 373 = −15350 J = −15.35 KJ
Answer is : Work is done by the system. Work done = −15.35 KJ
(ix) Calculate standard enthalpy of reaction, Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g), from the following data.
Δf H0(Fe2O3) = −824 kJ/mol,
Δf H0(CO) = −110 kJ/mol,
Δf H0(CO2) = −393 kJ/mol
Answer :
Given : Δf H0(Fe2O3) = −824 kJ/mol, Δf H0(CO) = −110 kJ/mol, Δf H0(CO2) = −393 kJ/mol
Δr H0= ?
Required equation,
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g) ….(1), ΔH1 = ?
Given equations :
2Fe(s) + O2(g) → Fe2O3(s) …..(2)
− − −
C(s) + O2(g) → CO(g) …….. (3) x 3
− − −
C(s) + O2(g) → CO2(g) …….. (4) x 3
+ + +
____________________________________
−2Fe(s) → −Fe2O3(s) − 3CO(g) + 3CO2(g)
∴ Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g) ….(1)
∴ eq(1) = − eq(2) − 3eq(3) + 3eq(4)
∴ Δr H0 = − Δ2 H0 − 3Δ3 H0 + 3Δ4 H0
∴ Δr H0 = − (−824) − 3(−110) + (−393) = −25 kJ
Answer is Δr H0 = −25 kJ
[/spoiler](x) For a certain reaction ΔH0 = 219 kJ and ΔS0 = −21 J/K. Determine whether the reaction is spontaneous or nonspontaneous.
Given: ΔH0 = 219 kJ; ΔS0 = −21 J/K = −21 x 10—3 kJ/K, ΔG° = ?
For standard state, T = 298 K
ΔG° = ΔH° — TΔS°
= 219 — 298 x (—21) x 10—3
= 219 + 6.258
= 225.3 kJ
Since ΔS < 0 and ΔG° > 0, the reaction is non spontaneous.
(xi) Determine whether the following reaction is spontaneous under standard state conditions. 2H2O(l) + O2(g) → 2H2O2(l) if ΔH0 = 196 kJ, ΔS0 = −126 J/K Does it have a cross—over temperature?
Given : 2H2O(l) + O2(g) → 2H2O2(l)
ΔH° = + 196 kJ
ΔS° = −126 JK−1 = 0.126 kJ K−1
T = 298 K
ΔG° = ?
Cross over temperature = T = ?
ΔG° = ΔH° — T ΔS°
= 196 — 298 (— 0.126)
= 196 + 37.55
= + 233.55 kJ
‘.’ ΔG° > 0, the reaction is non—spontaneous.
ΔH° > 0, ΔS° < 0,
Since at all temperatures, ΔG° > 0, there is no cross over temperature.
Answer is : The reaction is non—spontaneous.
There is no cross—over temperature for the reaction.
(xii) Calculate ΔU at 298 K for the reaction, C2H4(g) + HCl(g) → C2H5Cl(g), ΔH = −72.3 kJ How much PV work is done?
Given : C2H4(g) + HCl(g) → C2H5Cl(g), T = 298 K; ΔH = −72.3 kJ,
PV = ?, ΔU= ?
Δn = (moles of gaseous products) – (moles of gaseous reactants)
= 1− (1 + 1) = −1 mol
For PV work :
W= − ΔnRT
= − (−1) x 8.314 x 298 = 2478 J = 2.478 kJ
ΔH = ΔU + ΔnRT
∴ ΔU = ΔH − ΔnRT
= − 72.3 − (− 2.478)
= − 69.82 kJ
Answer is : PV work = 2.478 kJ
ΔU= − 69.82 kJ.
(xiii) Calculate the work done during synthesis of NH3 in which volume changes from 8.0 dm3 to 4.0 dm3 at a constant external pressure of 43 bar. In what direction the work energy flows?
Given : V1 = 8.0 dm3; V2 = 4.0 dm3; Pext = 43 bar
W = ? , What direction work energy flows?
W = −Pext(V2 − V1)
= − 43 (4 − 8)
= 172 dm3 bar
= 172 x 100]
= 17200 J
= 17.2 kJ
In this compression process, the work is done on the system and work energy flows into the system.
(xiv) Calculate the amount of work done in the
(a) oxidation of 1 mole HCl(g) at 200 0C according to reaction.
4HCl(g) + O2(g) → 2Cl2(g) + 2H2O(g)
(b) decomposition of one mole of NO at 300 0C for the reaction
2NO(g) → N2(g) + O2
Given :
(a) 4HCl(g) + O2(g) → 2Cl2(g) + 2H2O(g)
nHCl = 1mol, T = 273 + 200 = 473 K, W= ?
For 4 mol HCl
Δn = (2 + 2) − (4 + 1) = −1 mol
For 1 mo1 HCl Δn = − 1/4 = − 0.25 mol
W = − ΔnRT = − (−0.25) x 8.314 x 473 = 983.1 J
(b) Δn = (1 + 1) – 2 = 0 mol
W = –ΔnRT= – (0) x 8.314 x 473 = 0
Answer is : (a) W = 983.1 J
(b) W = 0.0 J.
(xv) When 6.0 g of O2 reacts with CIF as per 2ClF(g) + O2(g) → Cl2O(g) + OF2(g). The enthalpy change is 38.55 kJ. What is standard enthalpy of the reaction ?
Given : The given reaction is for 1 mol O2 or 32 g O2.
For 6.0 g O2 ΔH0 = 38.55 kJ
For32g O2 ΔH0 = \(\frac{32×38.55}{6}\) = 205.6 kJ
Answer is : ΔH° = 205.6 kJ.
(xvi) Calculate the standard enthalpy of formation of CH3OH(l) from the following data
(i) CH3OH(l)+ \(\frac{3}{2}\)O2(g) → CO2(g)+ 2H2O(l), ΔH0 = −726 kJ mol−1
(ii) C (Graphite) + O2(g) → CO2(g), ΔcH0 = −393 kJ mol−1
(iii) H2(g) + \(\frac{1}{2}\)O2(g) → H2O(l), ΔfH0 = −286 kJ mol−1
Given :
ΔC H0CH3OH = −726 kJ mol−1
ΔfH0CO2 = −393 kJ mol−1
ΔfH0H2O = −286 kJ mol−1
Δf H0CH3OH = ?
Required thermochemical equation :
C(s) + 2H2(g) + \(\frac{1}{2}\)O2(g) → CH3OH(l) …..(1) ΔH01 = ?
Given equations :
CH3OH(l) + \(\frac{3}{2}\)O2(g) → CO2(g) + 2H2O(l) …..(2) ΔH02
− − − −
C(graphite) + O2(g) → CO2(g) ….. (3) ΔH03
+
H2(g) + \(\frac{1}{2}\)O2(g) → H2O(l) ….. (4) ΔH04 x 2
+
Eq.(1) = − eq.(2) + eq.(3) + 2eq.(4)
∴ ΔH01 = −ΔH02 + ΔH02 + 2ΔH03
= − (−726) + (—293) + 2(—286)
= 726 − 393 − 572
= −239 kJ mol−1
Answer is : Standard enthalpy of formation = Δf H0 = −239 kJ mol−1
(xvii) Calculate ΔH0 for the following reaction at 298 K,
H2B4O7(s) + H2O(l) → 4HBO2(aq)
(i) 2H3BO3(aq) → B2O3(s) + 3H2O(l), ΔH0 = 14.4 kJ mol—1
(ii) H3BO3(aq) → HBO2(aq) + H2O(l) ΔH0 = −0.02 kJ mol—1
(iii) H2B4O7(s) → 2P2O3(s) + H2O(l), ΔH0 =17.3 kJ mol—1
Given :
Given equations :
2H3BO3(aq) → B2O3(s) + 3H2O(l) …..(1) ΔH01 = 14.4 kJ mol—1
H3BO3(aq) → HBO2(aq) + H2O(l) …..(2) ΔH02 = −0.02 kJ mol—1
H2B4O7(s) → 2P2O3(s) + H2O(l) .…..(3) ΔH03 =17.3 kJ mol—1
Required equation :
H2B4O7(s) + H2O(l) → 4HBO2(aq) .…..(4) ΔH04 = ?
To obtain eq.(4)
∴ eq.(4) = 4eq.(2) + eq.(3) – 2eq.(1)
∴ ΔH04 = 4ΔH02 + ΔH03 – 2ΔH01
= 4(−0.02) + 17.3 — 2(14.4)
= – 0.08 + 17.3 – 28.8
= – 11.58 kJ
Enthalpy change for the reaction = ΔrH0 = – 11.58 kJ
Answer is : ΔrH0 for the given reaction = – 11.58 kJ.
(xviii) Calculate the total heat required
(a) to melt 180 g of ice at 0 0C,
(b) heat it to 100 0C and then
(c) vapourise it at that temperature.
Given ΔfusH0(ice) = 6.01 kJ mol—1 at 0 0C, ΔvapH0( ) = 40.7 kJ mol—1 at 100 0C specific heat of water is 4.18 J g−1 K−1
Given : Mass of ice = m = 180 g
T1 = 273 + 0°C = 273K
T2 = 273 +100 °C = 373 K
ΔfusH0(ice) = 6.01 kJ mol−1
ΔvapH0( ) = 40.7 kJ mol−1
Specific heat of water = C = 4.18 J g−1 K−1
For converting 180 g ice into vapour, ΔHTotal = ?
Number of moles of H2O = 180/18 = 10 mol
The total process can be represented as,
H2O(s)10 mol \(\underleftrightarrow{ΔH_1}\) H2O(l) \(\underrightarrow{ΔH_2}\) H2O(l) \(\underrightarrow{ΔH_3}\) H2O(g)
(a) ΔH1 = ΔfusH0 =10 mol x 6.01 kJ mol−1 = 60.1 kJ
(b) When the temperature of water is raised from 0 °C to 100 °C (i.e., 273 K to 373 K), then
ΔH2 = m x C x ΔT
= m x C x (T2 − T1)
=180 g x 4.18 Jg−1 K−1 x (373 − 273) x 10−3 kJ = 75.24 kJ
(c) ΔH3 = ΔvapH = 10 mol x 40.7 kJ mol−1 = 407 kJ
Hence total enthalpy change,
ΔHTotal = ΔH1 + ΔH2 + ΔH3
= 60.1 + 75.24 + 407
= 542.34 kJ
Answer is : (a) ΔH1 =60.1 kJ (b) ΔH2 = 75.24 kJ (c) ΔHTotal = 542.34 kJ.
(xix) The enthalpy change for the reaction, C2H4(g) + H2(g) → C2H6(g) is −620 J when 100 ml of ethylene and 100 mL of H2 react at 1 bar pressure. Calculate the pressure volume type of work and ΔU for the reaction.
Given : C2H4(g) + H2(g) → C2H6(g)
100 mL 100 ml 100 ml
ΔH= − 620 J; V(C2H4) = 100 mL; V(H2) = 100 mL, Pext = 1 bar, W = ?, ΔU=?
ΔV = 100 − (100 + 100) = −100mL= −0.1 dm3
W = −Pext(V2 − V1)
= −Pext x ΔV
= −1 x (−0.1)
= 0.1 dm3bar
= 0.1 x 100 J
= +10 J
ΔH = ΔU + PΔV
ΔU = ΔH − PΔV = −620 − (+10) = −610 J
Answer is : W = +10 J, ΔU = −610 J
(xx) Calculate the work done and comment on whether work is done on or by the system for the decomposition of 2 moles of NH4NO3 at 100 0C
NH4NO3(s) → N2O(g) + 2H2O(g).
Given : NH4NO3(s) → N2O(g) + 2H2O(g).
n = 2 mol, T = 273 + 100 = 373 K,
W = ?, Comment on work = ?
Δng = (1 + 2) — 0 = 3 mol
For 1 mol of NH4NO3 Δng = 3 m
For 2 mol of NH4NO3 Δng = 6 mol
Due to 6 moles of gaseous products from 2 mol NH4NO3, there is work of expansion, hence work is done by the system.
W = − ΔnRT = −6 x 8.314 x 373
= −18606 J
= −18.61 KJ
Answer is : Work is done by the system
W= − 18.61 kJ.
PDF : Chapter-4-Chemical Thermodynamics-Text Book
PDF :Chapter-4-Chemical Thermodynamics- Notes
PDF :Chapter-4-Chemical Thermodynamics- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-3- Ionic Equilibria – Online Solutions
Next Chapter : Chapter-5-Electrochemistry – Online Solutions
We reply to valid query.