Transition and Inner transition Elements
Maharashtra Board-Class-12-Chemistry-Chapter-8
Notes-Part-3
Topics to be Learn : Part-3
|
Inner Transition (f-block) Elements: Lanthanoids and Actinoids :
- Elements in which differentiating electron enters into the pre-penultimate shell the (n − 2) f-orbital are known as f-block elements.
- They include 28 elements with atomic numbers ranging from 58 — 71 and atomic number 90 to 103, collectively.
- There are two f-series or two f-block elements, namely 4 f and 5 f series.
- The f-block includes two inner transition series namely the lanthanoid series, Cerium (58) to Lutetium (71) or the 4 f-block elements and the actinoid series, Thorium (90) to Lawrencium (103) or the 5f block elements.
- f-block elements are called inner transition elements since f orbital lies much inside the d-orbital in relation to the transition metals. These elements have 1 to 14 electrons in their f-orbital.
First inner transition elements :
- 4 f-block elements are called (first) inner transition elements and have partly filled inner orbitals or (4 f) orbitals.
- They have general outer electronic configuration (n − 2) f1−14, (n − 1) d0−1, ns2.
- There are two f-series, namely 4f (58Ce → 71Lu) and 5f (90Th → 103Lr) series, called lanthanoids and actinoids respectively.
- They show intermediate properties as compared to electropositive s-block elements and electronegative p-block elements. Hence they are called (first) inner transition elements.
Properties of f-block elements
- Properties are similar to d block elements
- Electrons are added to f subshells of (n-2) level
- Placed between (n-1)d and ns block elements
Lanthanoids or Lanthanoid series or Lanthanones : The series of fourteen elements from 58Ce to 71Lu in which a differentiating electron enters 4 f sub-shell and follows lanthanum is called lanthanoid series and the elements are called lanthanoids.
- They have general electronic configuration, [Xe] 4f1−14 5d0−1 6s2.
- They follow Lanthanum (Z = 57) in 3d— series.
Position of Lanthanoids in the periodic table :
- Group-3; Period-6.
- They interrupt the third transition series of d-block elements (i.e. 5 d series) in the sixth period.
- Actual position of lanthanoids is in between Lanthanum (Z = 57) and Hafnium (Z = 72).
- For the convenience they are placed separately below the main periodic table.
Their position is justified due to following reasons :
- All these elements have the same electronic configuration in ultimate and penultimate shells, one electron in 5d-orbital and two electrons in 6s-orbital.
- Group valence of all lanthanoids is 3.
- All lanthanoids from 58Ce to 7lLu have similar physical and chemical properties.
Rare earths :
- Lanthanoids or 4 f-block elements are called rare earths.
- Lanthanoids are never found in free state, and their minerals are not pure.
- They exhibit similar chemical properties hence cannot be extracted and separated by normal metallurgical processes.
- Lanthanoid metals are available on small scale. Therefore they are called rare earths.
| Similarities between transition and inner transition metals :
There are some properties similarity between transition and inner transition metals
|
Properties of lanthanoids :
- Lanthanoids are soft metals with silvery white colour. Colour and brightness reduces on exposure to air.
- They are good conductors of heat and electricity.
- Except promethium (Pm), all are non-radioactive in nature.
- The atomic and ionic radii decrease from La to Lu. (Lanthanoid contraction).
- They are paramagnetic.
- Coordination numbers are greater than 6.
- They become ferromagnetic at lower temperature.
- Their magnetic and optical properties are independent of environment.
- They are called rare earths as their extraction was difficult.
- They are abundant in earth’s crust.
- All Lanthanoids form hydroxides which are ionic and basic. Basicity decreases with atomic number,
- They react with nitrogen to give nitrides and with halogen to give halides.
- 2Ln + N2 \(\underrightarrow{Δ}\) 2LN
- Ln + 3X2 \(\underrightarrow{Δ}\) 2Ln x X3 (X-halogen)
- When lanthanoids are heated at elevated temperatures (~ 2800 K) with carbon, the carbides with general formula LnC2 are obtained.
Ionisation enthalpy of lanthanoids :
- The first ionisation enthalpy of lanthanoids is nearly same. It is very high for Gd and Yb
- The ionisation enthalpy increases from first (IE1) to third (IE3).
| Lanthanoid | IE1 | IE2 | IE3 |
| La | 538.1 | 1067 | 1850.3 |
| Ce | 528.0 | 1047 | 1949 |
| Pr | 523.0 | 1018 | 2086 |
| Nd | 530.0 | 1034 | 2130 |
| Pm | 536.0 | 1052 | 2150 |
| Sm | 543.0 | 1068 | 2260 |
| Eu | 547.0 | 1085 | 2400 |
| Gd | 592.0 | 1170 | 1990 |
| Tb | 564.0 | 1112 | 2110 |
| Dy | 572.0 | 1126 | 2200 |
| Ho | 581.0 | 1139 | 2200 |
| Er | 589.0 | 1151 | 2190 |
| Tm | 596.7 | 1163 | 2284 |
| Yb | 603.4 | 1175 | 2415 |
| Lu | 523.5 | 1340 | 2022 |
Electronic configuration :
- The electronic configuration of lanthanoids is [Xe] 4f0-14 5d0-2 6s2.
- This is because 1s22s22p63s23p64s23d104p65s24d105p6 is the electronic configuration of xenon and we can simplify the electronic configuration of lanthanoids by [Xe] 4f0-14 5d0-2 6s2
- In lanthanoids, the differentiating electron enters prepenultimate shell, 4f
Important features of the electronic configuration of lanthanoids :
- Lanthanoids show two types of electronic configurations : (a) an expected or idealized (b) an observed electronic configuration.
- In the idealized electronic configuration, the filling of the 4f -orbitals is regular but in the observed configuration there is the shift of a single electron from 5d to 4f sub-shell.
- Lanthanum (57) has an electronic configuration [Xe] 4f O 5d ' 6s2. It does not have any f-electron.
- The next incoming electron does not enter the 5d sub-shell but goes to the 4f sub-shell.
- 14 electrons are progressively filled in the 4f sub-shell as the atomic number increases by one unit from La to Lu.
- La, Gd and Lu are the only elements which possess one electron in a 5d orbital, while in all other lanthanoids. The 5d sub-shell is empty.
- La-(4f 0), Gd-(4f 7) and Lu-(4f 14) possess extra stability due to their empty, half-filled and completely filled 4 f-orbitals respectively.
- The 4f-electrons in the prepenultimate shell are shielded by the outermost higher orbitals, 5s2, 5p°, 5d1, 6s2, i.e_ by eleven electrons, hence they are less effective in chemical bonding.
Electronic configuration and atomic ionic radii of lanthanoids :
| Element | Symbol | Atomic number | Electronic configuration | |
| Expected (ground state) | Observed (ground state) | |||
| Lanthanum | La | 57 | [Xe]4f05d16s2 | [Xe]4f05d16s2 |
| Cerium | Ce | 58 | [Xe]4f26s2 | [Xe]4f15d16s2 |
| Praseodymium | Pr | 59 | [Xe]4f36s2 | [Xe]4f36s2 |
| Neodymium | Nd | 60 | [Xe]4f46s2 | [Xe]4f46s2 |
| Promethium | Pm | 61 | [Xe]4f56s2 | [Xe]4f56s2 |
| Samarium | Sm | 61 | [Xe]4f66s2 | [Xe]4f66s2 |
| Europium | Eu | 63 | [Xe]4f76s2 | [Xe]4f76s2 |
| Gadolinium | Gd | 64 | [Xe]4f86s2 | [Xe]4f75d16s2 |
| Terbium | Tb | 65 | [Xe]4f96s2 | [Xe]4f96s2 |
| Dysprosium | Dy | 66 | [Xe]4f106s2 | [Xe]4f106s2 |
| Holmium | Ho | 67 | [Xe]4f116s2 | [Xe]4f116s2 |
| Erbium | Er | 68 | [Xe]4f126s2 | [Xe]4f126s2 |
| Thulium | Tm | 69 | [Xe]4f136s2 | [Xe]4f136s2 |
| Ytterbium | Yb | 70 | [Xe]4f146s2 | [Xe]4f146s2 |
| Lutetium | Lu | 71 | [Xe]4f145d16s2 | [Xe]4f145d16s2 |
Oxidation state :
- The common oxidation state of the Lanthanoids is +3 due to the loss of 2 electrons from outermost 6s orbital and one electron from the penultimate 5d sub-shell.
- Gd3+ and Lu3+ show extra stability due to their half filled and completely filled f-orbitals,
Gd3+ = [Xe]4f7
Lu3+ = [Xe] 4f14
- Ce and Tb attain the 4f0 and 4f7configurations in the 4+ oxidation states. Eu and Yb attain the 4f7 and 4f14 configurations in the 2 + oxidation states. Sm and Tm also show the 2+ oxidation state although their stability can be explained based on thermodynamic factors.
- Some lanthanoids show 2 + and 4 + oxidation states even though they do not have stable electronic configuration of 4f0, 4f7 or 4f14
- E,g. Pr4+ (4f1),Nd2+ (4f4),Sm2+ (4f6),Dy4+ (4f8) etc.
Colour and Spectra :
- The colour in lanthanoid ions is due to the presence of unpaired electrons in partially filled 4 f sub-shells.
- Due to the absorption of radiations in the visible region there arises the excitations of the unpaired electrons from f-orbital of lower energy to the f-orbital of higher energy giving f → f transitions.
- The observed colour is complementary to the colour of the light absorbed.
- The colour of tripositive ions (M3 +) depends upon the number of unpaired electrons in f-orbitals. Hence thelanthanoid ions having equal number of unpaired electrons have similar colour.
- The colours of M3 + ions of the first seven lanthanoids, La +3 to Eu3+ are similar to those of seven elements Lu3+ to Tb3+ in the reverse order.
- The colour of ions having nf electrons is about the same as those having (14-n)f electrons. (where n is an integer 1-13).
| Ln ion | No. of f-electrons | Colour | |
| Pr3+ | 4f 2 | green | (14 -n) f-electrons =14-2 =12 |
| Tm3+ | 4f 12 | green | n f-electrons =12 |
| Nd3+ | 4f 3 | pink | (14 -n) f-electrons =14-3 =11 |
| Er3+ | 4f 11 | pink | nf-electrons =11 |
Colourless ions :
Ce3+ ion is colourless :
- The electronic configuration of Ce3+ is, [Xe] 4f 1
- Even though there is one unpaired electron in 4f sub-shell, the f → f transition involves very low energy. Hence, Ce3+ion does not absorb radiation in the visible region. Therefore Ce3+ ion is colourless.
Gd3+ is colourless :
- Gd3+ has electronic configuration, [Xe] 4f 7
- Due to extra stability of half filled orbital, it does not allow f → f transition, and hence does not absorb radiations in the visible region. Hence Gd3+is colourless.
The salts of (i) La3+ and (2) Lu3+ are colourless :
- La3+ has electronic configuration, [Xe] 4 f 0. Since there are no unpaired electrons in 4 f-orbital, f → f transition is not possible. Hence La3+ ions do not absorb radiations in visible region, and they are colourless.
- Lu3+ has electronic configuration [Xe] 4f 14 . Since there are no unpaired electrons in 4 f- orbital, f → f transition is not possible. Hence Lu3+ ions do not absorb radiations in visible region and they are colourless.
Atomic and ionic radii (Lanthanoid Contraction) :
Lanthanoid Contraction :Atomic and ionic radii get smaller as we progress through the lanthanoid series. This steady decrease in the atomic and ionic radii is called Lanthanoide contraction.
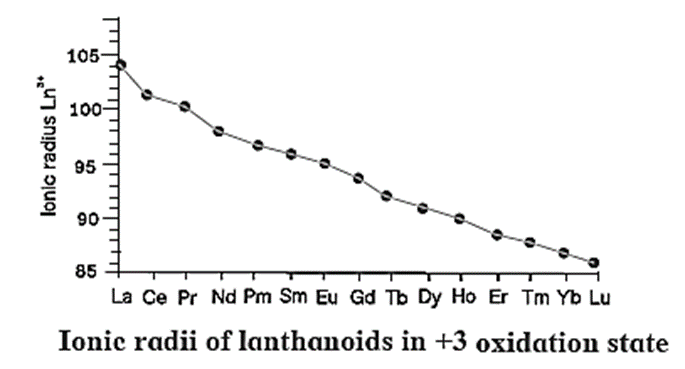
Factors of lanthanoid contraction :
- As the atomic number of lanthanoids or 4 f-block elements increases the positive nuclear charge increases and correspondingly electrons are added to the prepenultimate 4 f sub-shell.
- The attraction of nucleus on 4 f-electrons increases with the increase in atomic number.
- The outer eleven electrons namely, 5s2, 5p6, 5d1 and 6s2 do not shield inner 4 f-electrons from the nucleus.
- There is imperfect shielding of each 4 f-electron from other 4 f-electrons.
- As compared to d sub—shell, the extent of shielding for 4 f-electrons is less.
- Due to these cumulative effects, 4 f-electrons experience greater nuclear attraction and hence valence shell is pulled towards the nucleus to the greater extent decreasing atomic and ionic radii appreciably.
- From 57La to 58Ce there is a sudden contraction in atomic radius from 187 pm to 183 pm but the further decrease up to the last 4f-element, 71Lu is comparatively low (about 10 pm).
- Hence all lanthanoids have similar properties. Therefore they cannot be separated from each other easily by normal metallurgical methods but require special methods.
Effect of Lanthanoid Contraction
The lanthanoid contraction has a definite effect on the properties of lanthanoids as well as on the properties of post-lanthanoid elements.
Decrease in basicity :
- In lanthanoids due to lanthanoid contraction, as the atomic number increases, the size of the lanthanoid atoms and their tripositive ions decreases, i.e. from La3+ to Lu3+.
- As size of the cation decreases, according to Fajan’s rule, the polarizability increases and thus the covalent character of the M-OH bond increases, and ionic character decreases.
- Therefore the basic nature of the hydroxides decreases.
- Basicity and ionic character decrease in the order La(OH)3 > Ce(OH)3 > Lu(OH)3.
Ionic radii of post-lanthanoids :
Elements following the lanthanoids in the 6th period (third transition series, i.e. 5d-series) are known as post-lanthanoids.
- Due to lanthanoid contraction the atomic radii (size) of elements which follow lanthanum in the 6th period (3rd transition series - Hf, Ta, W, Re)—are similar to the elements of the 5th period (4d-series Zr, Nb Mo, Tc).
- Due to similarity in their size, post-lanthanoid elements (Sd-series) have closely similar properties to the elements of the 2nd transition series (4d-series) which lie immediately above them.
- Pairs of elements namely Zr-Hf(Gr-4), Nb-Ta(Gr-5 ), Mo-W(Gr-6), Tc-Re(Gr-7) are called chemical twins since they possess almost identical sizes and similar properties.
Effective magnetic moments of lanthanoids in +3 oxidation state :
| Ln | Ln3+ oxidation state | No. of unpaired electrons | Observed magnetic moment, μeff B.M. |
| La | 4f0 | 0 | 0 |
| Ce | 4f1 | 1 | 2.3-2.5 |
| Pr | 4f2 | 2 | 3.4-3.6 |
| Nd | 4f3 | 3 | 3.5-3.6 |
| Pm | 4f4 | 4 | -- |
| Sm | 4f5 | 5 | 1.4-1.7 |
| Eu | 4f6 | 6 | 3.3-3.5 |
| Gd | 4f7 | 7 | 7.9-8.0 |
| Tb | 4f8 | 6 | 9.5-9.8 |
| Dy | 4f9 | 5 | 10.4-10.6 |
| Ho | 4f10 | 4 | 10.4-10.7 |
| Er | 4f11 | 3 | 9.4-9.6 |
| Tm | 4f12 | 2 | 7.1-7.6 |
| Yb | 4f13 | 1 | 4.3-4.9 |
| Lu | 4f14 | 0 | 0 |
Lu3+ has observed magnetic moment zero. Since magnetic moment is zero, it has no unpaired electrons.
Applications of lanthanoids :
The lanthanoid compounds are present in every household.
- Lanthanoid compounds are used inside the colour television tubes and computer monitor. For example, mixed oxide (Eu, Y)2O3 releases an intense red colour when bombarded with high energy electrons.
- Lanthanoid ions are used as active ions in luminescent materials. (Optoelectronic application)
- Nd : YAG laser is the most notable application. (Nd : YAG = neodymium doped ytterium aluminium garnet)
- Erbium doped fibre amplifiers are used in optical fibre communication systems.
- Lanthanoids are used in cars, superconductors and permanent magnets.
Actinoids :
- The last row of elements in the periodic table is the actinoid series.
- Series of 14th elements from thorium (Z =72) to lawrencium (Z=103), which follow actinium (89Ac) and in which differentiating electrons are progressively filled in 5 f-orbitals in prepenultimate shell are called actinoids.
- Actinoids are 5 f-series elements in which electrons progressively enter into 5f-orbitals, which are inner orbitals.
- Their general electronic configuration is, [Rn]86 5f1-14 6d0-1 7s2.
- They show intermediate properties as compared to electropositive s-block elements and electronegative p-block elements. Hence they are called second inner transition elements.
- Position of actinoids in the periodic table : Group — 3; Period — 7
- For the convenience they are placed separately below the periodic table.
- The long lived isotopes such as thorium, protactinium, uranium, neptunium, plutonium and americium have high densities (~ 15-20 g cm-3), high melting points (~1000 oC) and high boiling points (~3000 oC).
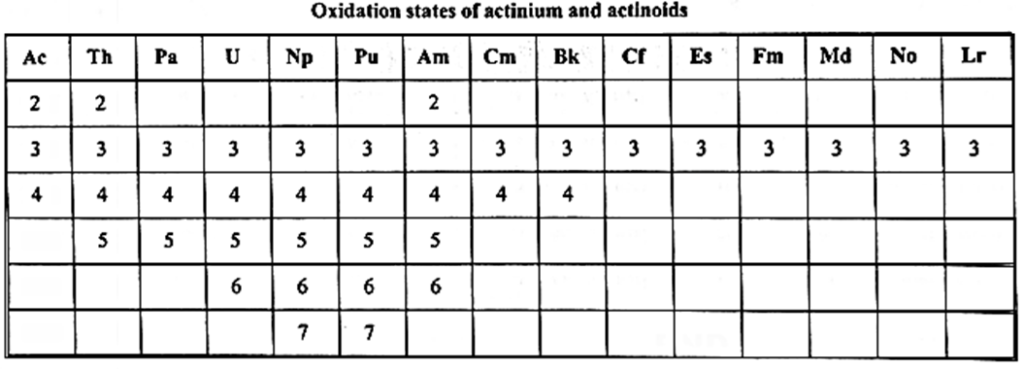
Oxidation states of actinoids : .
- Due to availability of electrons in 5f, 6d and 7s sublevels, lanthanoids show varied oxidation states.
- The most common oxidation state is + 3 due to loss of one electron from 6d and two electrons from 6s-orbitals.
- Ac, Th and Am show + 2 oxidation state.
- Th, Pa, U, Np, Pu, Am and Cm show + 4 oxidation state.
- Np and Pu show the highest oxidation state + 7.
- U, Np, Bk, Cm and Am show stable oxidation state + 4.
- In + 6 oxidation state, due to high charge density the actinoid ions form oxygenated ions, e. g. UO2+, NpO2+ etc.
Actinoids show variable oxidation states :
- The large number of variable oxidation states of actinoids is due to very small energy difference between 5f, 6d and 7s subshells.
- Due to the loss of three electrons from 6d1 and 7s2, the common oxidation state is + 3 but due to further loss of electrons from 5f subshell, actinoids show higher oxidation states.
- The variable oxidation states are + 2 to + 7.
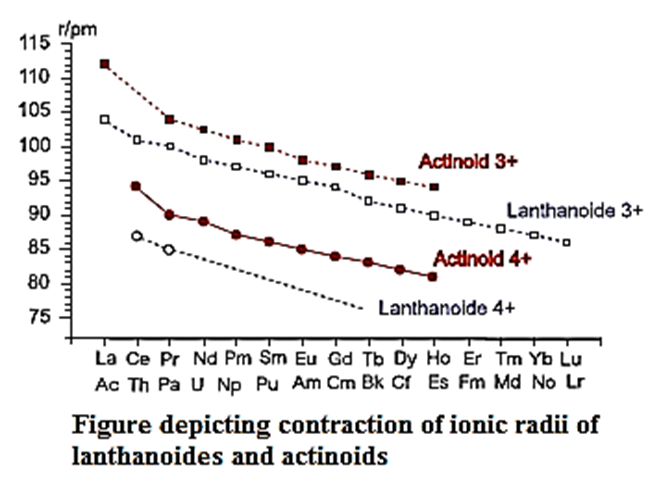
Actinoid contraction: The gradual decrease in atom' and ionic radii of actinoids with the increase in atomic number is called actinoid contraction.
The extent of actinoid contraction is greater than lanthanoid contraction because of the following reasons :
The electronic configurations of :
Lanthanoids [Xe] 4f1−14 5d0−1 6s2
Actinoids : [Rn] 5f1−14 6d0−1 7s2
- The mutual screening offered in case of 5f-electrons is less than that in the 4f-electrons.
- Hence, the outer orbitals are pulled to the greater extent by nuclei in actinoids (5f-series) than in lanthanoids (4f-series).
- Therefore, actinoid contraction is greater than lanthanoid contraction.
Properties of actinoids :
- Actinoids are silvery white (similar to lanthanoids).
- They are highly reactive radioactive elements.
- Most of these elements are not found in nature. They are radioactive and man-made.
- They experience decrease in the atomic and ionic radii from Ac to Lw, known as actinoid contraction.
- The common oxidation state is + 3. Elements of the first half of the series exhibit higher oxidation states.
Applications of actinoids :
- Thorium oxide (ThO2) with 1% CeO2 is used as a major source of indoor lighting, as well as for outdoor camping.
- Uranium is used in the nuclear reactors.
- The isotopes of Thorium and Uranium have very long half-life, so that we get very negligible radiation from them:
- Hence they can be used safely.
Electronic configuration of actinoids and their ionic radii in +3 oxidation state :
| Element | Symbol | Atomic number | Electronic configuration | *Atomic radii, pm | *Ionic radii (Ac3+), pm | |
| ground state | +3 oxidation state | |||||
| Actinium | Ac | 89 | [Rn]5f 06d17s2 | 5f 0 | 203 | 126 |
| Thorium | Th | 90 | [Rn]5f 06d27s2 | 5f 1 | 180 | - |
| Protactinium | Pa | 91 | [Rn]5f 26d17s2 | 5f 2 | 162 | 118 |
| Uranium | U | 92 | [Rn]5f 36d17s2 | 5f 3 | 153 | 118 |
| Neptunium | Np | 93 | [Rn]5f 46d17s2 | 5f 4 | 150 | 116 |
| Plutonium | Pu | 94 | [Rn]5f 66d07s2 | 5f 5 | 162 | 115 |
| Americium | Am | 95 | [Rn]5f 76d07s2 | 5f 6 | 173 | 114 |
| Curium | Cm | 96 | [Rn]5f 76d17s2 | 5f 7 | 174 | 112 |
| Berkelium | Bk | 97 | [Rn]5f 96d07s2 | 5f 8 | 170 | 110 |
| Californium | Cf | 98 | [Rn]5f 106d07s2 | 5f 9 | 186 | 109 |
| Einsteinium | Es | 99 | [Rn]5f 116d07s2 | 5f 10 | 186 | 98 |
| Fermium | Fm | 100 | [Rn]5f 126d07s2 | 5f 11 | 198 | 91 |
| Mendelevium | Md | 101 | [Rn]5f 136d07s2 | 5f 12 | 194 | 90 |
| Nobelium | No | 102 | [Rn]5f 146d07s2 | 5f 13 | 197 | 95 |
| Lawrencium | Lr | 103 | [Rn]5f 146d17s2 | 5f 14 | 171 | 88 |
Similarities and differences between lanthanides and actinoids :
Similarities :
- Both the series show a +3 oxidation state.
- In both the series, the f-orbitals are filled gradually.
- Ionic radius of the elements in both series decreases with an increase in atomic number.
- The electronegativity of all the elements in both the series is low and are said to be highly reactive.
- The nitrates, perchlorates and sulphates of all the elements are soluble while the hydroxides, fluorides and carbonates are insoluble
Differences
| Lanthanoids | Actinoids |
| Lanthanoids show a maximum oxidation state of +4 | Actinoids show oxidation states of +3, +4, +5, +6 and +7 |
| Lanthanoids do not form complexes easily. | Actinoids have a greater tendency to form complexes with ligands such as thio-ethers |
| All lanthanoids are non-radioactive except promethium | Actinides are radioactive in nature |
| Lanthanoids do not form oxocations. | Actinides form oxocations such as UO+, PuO+, NpO+2 |
| Most of the lanthanoids are colourless in nature, | The actinoids are coloured ions |
Know This :
| Uranium is another actinoid which is in great demand as it is used in the nuclear reactors. One of the extraction methods for uranium has a very interesting chemistry. The ore containing U(IV) oxide, UO2, is first treated with Fe(III) ion to give U(VI) oxide, UO3
UO2(s) + H2O(l) → UO3 (s) + 2H+ (aq) + 2e− Fe3+ (aq) + e− → Fe2+(aq) Addition of H2SO4 to this solution produces uranyl sulphate containing UO22+ cation: UO3(s) + H2SO4(aq) → UO2SO4(aq) + H2O(l) After purification, ammonia is added to the solution giving bright yellow precipitate of ammonium diuranate, (NH4)2U7O7: 2UO2SO4(aq) + 6NH3(aq) + 3H2O(l) → (NH4)2U7O7(s) + 2(NH4)2SO4(aq) This yellow cake is the marketable form of uranium! |
Transuranic elements :
- The man-made elements heavier than Uranium (Z = 92) in the Actinoid series are called transuranic elements.
- These are synthetically or artificially prepared (man-made) elements starting from Neptunium (Z = 93).
- Transuranic elements are generally considered to be from Neptunium (Z = 93) to Lawrencium (Z = 103 ). Recently elements from atomic number 104 (Ri) to atomic number 118 (Og) or (Uuo) in 6 d series, have also been identified as transuranic elements.
- All transuranic elements are radioactive.
Some comparison between Pre-Transition, Lanthanoids and Transition Metals :
| Pre-Transition Metals | Lanthanoids | Transition Metals |
| Essentially monovalent - show group (n+) oxidation state | Essentially in (+3) oxidation state (+2/+4 for certain configurations) | Show variable oxidation states |
| Periodic trends dominated by effective nuclear charge at noble gas configuration | Lanthanoid contraction of Ln3+ | Size changes of Mn, less marked |
| Similar properties for a given group | Similar properties | Substantial changes in properties |
| Always 'hard' (O, X, N donors, preferably negatively charged) | Always 'hard' (O, X, N donors, preferably negatively charged) | Heavier metals (increasingly from Fe-Cu) may show a 'soft' character |
| No ligand field effects | Insignificant ligand field effects | Substantial ligand field effects |
| Poor coordination properties (C.N. determined by size) | High coordination numbers (C.N. determined by size) | Coordination number 6
is typical maximum (many exceptions) |
| Flexibility in geometry | Flexibility in geometry | Fixed geometries (ligand field effects) |
| No magnetism | Show magnetism | Show magnetism |
Post actinoid elements :
- Elements from atomic number 104 to 118 are called post actinoid elements.
- The post actinoid elements known so far are transition metals.
- They can be synthesised in the nuclear reactions.
- As they have very short half life period, it is ditficult to study their chemistry.
- Rutherfordium forms a chloride (RfCl4) similar to zirconium and hafnium in + 4 oxidation state.
- Dubnium resembles niobium and protactinium.
Know This :
| Traditionally, no element was named after a still-living scientist.
This principle was put to an end with naming the element 106 as ‘Seaborgium’. |
PDF : Chapter-8-Transition and Inner transition Elements-Text Book
PDF : Chapter-8-Transition and Inner transition Elements- Notes
PDF : Chapter-8-Transition and Inner transition Elements- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-8-Elements of Groups 16, 17 and 18 – Online Notes
Next Chapter : Chapter-9-Coordination Compounds – Online Notes
We reply to valid query.