Elements of Groups 16, 17, and 18
Maharashtra Board-Class-12-Chemistry-Chapter-7
Notes-Part-3
Topics to be Learn : Part-3
|
Chlorine and compounds of chlorine :
Chlorine :
- Chlorine was discovered by Scheele, a German Swedish chemist in 1774 by the action of HCl on MnO2. In 1810, Davy established its elementary nature and suggested the name chlorine on account of its colour. (Greek, Chloros = greenish yellow).
Preparation :
(i) Chlorine obtained from HCl :
Chlorine is obtained by the oxidation of hydrochloric acid by various oxidising agent :
- Manganese dioxide :
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
- Potassium permanganate :
2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2
(ii) Chlorine obtained from ruck salt or NaCl :
Chlorine can also be prepared by the action of concentrated sulfuric acid on a mixture of sodium chloride (common salt) and manganese dioxide. The reaction takes place in two steps.
Step 1 : 4NaCl + 4H2SO4 → 4NaHSO4 + 4HCl
Step2 : MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Final reaction : 4NaCl + MnO2 + 4H2SO4 → 4NaHSO4 + MnCl2 + 2H2O + Cl2
Manufacture of chlorine :
(i) Deacon’s process :
Chlorine is manufactured by the oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 as catalyst at 723 K.
4HCl + O2 \(\underrightarrow{CuCl_2}\) 2Cl2 + 2H2O
(ii) Electrolytic process :
- On a large scale chlorine is obtained by electrolytic process.
- The electrolyte is brine, a concentrated solution of NaCl.
- Nelson’s two compartments diaphragm cell is used for electrolysis in which stout graphite rod is used as an anode while U shaped steel vessel is used as a cathode.
- By the electrolysis of brine (concentrated NaCl solution), chlorine is liberated at the anode.
NaCl ⇌ Na+ + Cl
Cathode: 2H2O + 2e− → H2 + 2OH−
Na+ + OH− → NaOH
Anode : Cl− → Cl + e−
Cl + Cl → Cl2
Physical Properties of Chlorine :
- Chlorine is a greenish-yellow gas having pungent and suffocating odour.
- It is poisonous in nature.
- It can be easily liquified into a greenish yellow liquid, which boils at 293 K.
- It dissolves in water to give chlorine water.
- It is 2-5 times heavier than air.
Chemical properties of chlorine :
(i) Reaction with metals :
- Chlorine reacts with metals to form chlorides.
2Al + 3Cl2 → 2AlCl3
2Na + Cl2 → 2NaCl
2Fe + 3Cl2 → 2FeCl3
(ii) Reaction with nonmetals :
- Chlorine reacts with nonmetals to form their chlorides.
P4 + 6Cl2 → 4PCl3
S8 + 4Cl2 → 4S2Cl2
(iii) Affinity for hydrogen :
- Chlorine has great affinity for hydrogen. It reacts with hydrogen and compounds containing hydrogen to form HCl.
H2 + Cl2 → 2HCl
H2S + Cl2 → HCl + S
(iv) Reaction with NH3 :
- Chlorine when reacted with excess of ammonia gives ammonium chloride and nitrogen.
8NH3(Excess) + 3Cl2 → 6NH4Cl + N2
- Excess of chlorine reacts with ammonia to give nitrogen trichloride (explosive).
NH3 + 3Cl2(Excess) → NCl3 + 3HCl
(v) Reaction with alkali :
- Chlorine reacts with cold and dilute alkali to produce a mixture of chloride and hypochlorite. When reacted with hot concentrated alkali, chloride and chlorate are produced.
2NaOH(Cold and dilute) + Cl2 → NaCl + NaOCl + H2O
6NaOH(Hot and conc.) + 3Cl2 → 5NaCl + NaClO3 + 3H2O
- Chlorine when reacted with dry slaked lime gives bleaching powder.
2Ca(OH)2 + 2Cl2 → Ca(OCl) 2 + CaCl2 + 2H2O
(vi) Reaction with hydrocarbons :
- Chlorine reacts with saturated hydrocarbons to give substitution products and with unsaturated hydrocarbons gives addition products.
- With methane, chlorine forms substituted products.
CH4 + Cl2 \(\underrightarrow{UV}\) CH3Cl + HCl
CH3Cl + Cl2(Excess) → CH2Cl2 + HCl
CH2Cl2 + Cl2 → CHCl3 + HCl
CHCl3 + Cl2 → CCl4 + HCl
- With unsaturated hydrocarbons, chlorine forms addition products.
CH2=CH2 + Cl2 (Ethylene) \(\underrightarrow{room\,\,temp.}\) CH2Cl−CH2Cl ( Ethylene dichloride)
(vii) Oxidising property :
- Chlorine oxidises ferrous salts to ferrric salts and sulfites to sulfates.
2FeSO4 + H2SO4 + Cl2 → Fe2(SO4)3 + 2HCl
Na2SO3 + Cl2 + H2O → Na2SO4 + 2HCl
- It oxidises sulfur dioxide to sulfur trioxide and iodine to iodate. In presence of water they form sulfuric acid and iodic acid respectively.
SO2 + 2H2O + Cl2 → H2SO4 + 2HCl
I2 + 6H2O + 5Cl2 → 2HIO3 + 10HCl
(viii) Bleaching Property :
- Chlorine requires the presence of moisture (water) for bleaching.
- It liberates nascent oxygen from water which is responsible for its oxidising and bleaching property
Cl2 + H2O → HCl + HOCl
HOCl → HCl + [O]
- Chlorine bleaches vegetable matter or coloured organic matter in the presence of moisture to colourless matter.
Coloured organic matter + [O] → Colourless organic matter
- Since chlorine has an ability of killing harmful micro-organisms, it acts as a good disinfecting agent.
- This ability is due to the oxidising nature of chlorine which produces nascent oxygen in aqueous solutions.
Uses of Chlorine :
- For purification (sterilizing) of drinking water.
- For bleaching wood pulp required for manufacture of paper and rayon, bleaching cotton and textiles.
- For extraction of metals like gold and platinum.
- In the manufacture of dyes, drugs and organic compounds such as CCl4, CHCl3,
- DDT, refrigerants, etc.
- In the preparation of poisonous gases such as phosgene (COCl2), tear gas (CCl3NO2), mustard gas (ClCH2CH2SCH2CH2Cl).
| Know This :
Bleaching by chlorine is permanent. It bleaches cotton fabrics, wood pulp, litmus, etc. However chlorine is not used to bleach delicate materials such as silk, wool etc. as it is a strong bleaching and oxidising agent. This dual action will damage the base material. |
Hydrogen Chloride :
- Hydrogen chloride was prepared by Glauber in 1648 by heating common salt with concentrated sulfuric acid. Davy in 1810 showed that it is a compound of hydrogen and chlorine.
Preparation of Hydrogen Chloride:
- In the laboratory, hydrogen chloride is prepared by heating sodium chloride (common salt) with concentrated sulfuric acid.
NaCl + H2SO4 \(\underrightarrow{470\,K}\) NaHSO4 + HCl
NaHSO4 + NaCl \(\underrightarrow{470\,K}\) Na2SO4 + HCl
- HCl gas can be dried by passing it through concentrated sulfuric acid and then collected by upward displacement of air.
Physical properties of HCl
- Hydrogen chloride is a colourless and pungent smelling gas.
- It is heavier than air.
- It can be easily liquified to a colourless liquid (b.p. 189 K) which freezes to a white crystalline solid (m.p. 159 K)
- It is highly soluble in water.
Chemical properties :
(i) Acidic property :
- Hydrogen chloride is highly soluble in water and ionises as follows :
HCl(aq) + H2O → H3O+(aq) + Cl−(aq) Ka = 107
- The aqueous solution of HCl gas is called hydrochloric acid. High value of dissociation constant (Ka) indicates that it is a strong acid in water.
(ii) Reaction with NH3 :
- Hydrochloric acid reacts with ammonia and gives white fumes of ammonium chloride.
NH3 + HCl → NH4Cl
(iii) Reaction with noble metals :
- When three parts of concentrated HCl and one part of concentrated HNO3 are mixed, aqua regia is formed.
- Noble metals like gold, platinum get dissolved in aqua regia.
Au + 4H+ + NO3− + 4Cl− → AuCl4− + NO + 2H2O
3Pt + 16 H+ + 4NO3− + 18Cl → 3PtCl62− + 4NO + 8H2O
Uses of hydrogen chloride (hydrochloric acid) :
- in the manufacture of chlorine and ammonium chloride,
- to manufacture glucose from corn, starch
- to manufacture dye
- in medicine and galvanising
- as an important reagent in the laboratory
- to extract glue from bones and for the purification of bone black.
- for dissolving metals,
Fe + 2HCl(aq) → FeCl2 + H2(g)
Interhalogen compounds :
Interhalogen compounds : Compounds formed by the combination of atoms of two different halogens are called interhalogen compounds.
- In an interhalogen compound, of the two halogen atoms, one atom is more electropositive than the other.
- The interhalogen compound is regarded as the halide of the more electropositive halogen. For example, ClF, BrF3, ICl
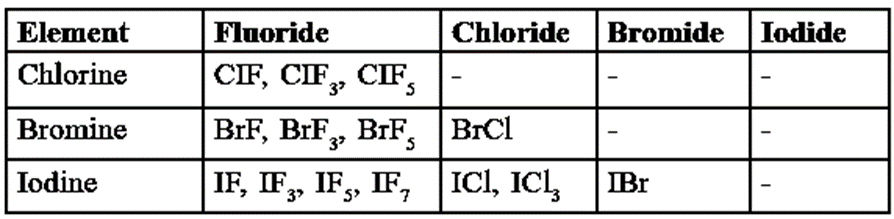
Various types of interhalogen compounds :
| Know This :
• The ions formed by combination of different halogens are called polyhalide ions or interhalogen ions, For example, K+[Cl3− ], [NH4]+ [I5] − , which contain Cl3− and I5− ions. • ICl Iodine monochloride in glacial acetic acid called Wijs solution is used in determination of iodine value of an oil. |
Classification : Depending on their composition, interhalogen compounds are classified into four types.
- The general compositions of interhalogen compounds are XX’, XX’3, XX’5 and XX’7 where halogen X is more electropositive and has larger size than another halogen X’. For example,
| Type | Example |
| XX’ | CIF, BrF, BrCl, ICl, IBr |
| XX’3 | CIF3, BrF3, IF3, ICl3 (unstable) |
| XX’5 | CIF5, BrF5,IF5 |
| XX’7 | IF7 |
- As the ratio of radii of X and X’ increases, the number of atoms oft X’ per molecule of inter-halogen compound increases. For example, iodine having the largest size with fluorine of the smallest size forms stable IF7.
General characteristics of interhalogen compounds :
- The compound is considered as the halide of X. For example, ClF. Here the halogen having larger size is chlorine, it is more electropositive than F and hence the interhalogen compound is named as chlorine monofluoride. (n) is the number of atoms of X' attached to X. As the ratio [radius of X : radius of X'] increases the value of n also increases.
- Interhalogen compounds have even number of atoms 2, 4, 6, 8. For example, ClF3 has 4 atoms.
- The properties of interhalogen compounds are generally intermediate between those of the halogens from which they are made.
- The central halogen exhibits different oxidation states in different interhalogen compounds.
- Number of X' atoms in the compounds is always odd.
- They are all diamagnetic .
- Since the electronegativity difference between two different halogens is low, the interhalogen compounds are covalent in nature.
- Since the interhalogen bond (X—X’) is weaker than parent halogens, they are more reactive than halogens.
| Know This :
• XX' compounds are more reactive than X2 or X'2. In the X-X' bond X' is more electronegative than X, while in X2 and X'2 both atoms have same electronegativity, hence the X-X' bond energy is less than the X-X or X'-X' bond energy. |
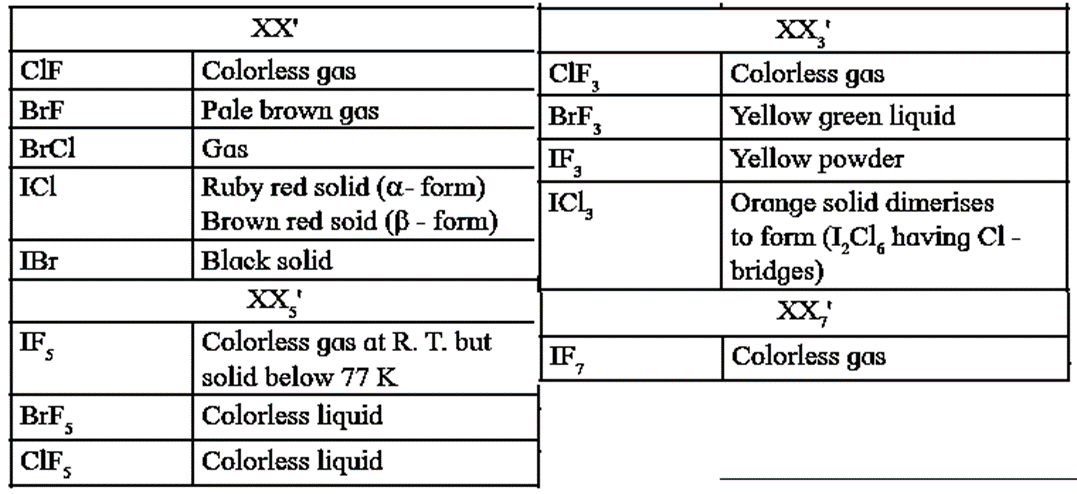
Physical States of Interhalogen compounds at 25°C
Methods of Preparation of Interhalogen compounds :
All interhalogen compounds are prepared by direct reaction of halogens or reaction of halogen on lower interhalogen compounds. The compound formed depends upon specific conditions of the reactions.
(i) By direct combination :
Chlorine monofluoride, CIF : It is a colourless as obtained by reacting equal volumes of Cl2 and F2.
Cl2 + F2 → 2ClF
.Chlorine trifluoride, ClF3 : It is a colourless gas, obtained by reacting Cl2 with excess of F2.
Cl2 + 3F2(excess) → 2ClF3
Bromine trifluoride, BrF3 : It is a yellow green liquid obtained by reacting Br2 With excess of F2.
Br2 + 3F2(excess) → 2BrF3
Iodine trichloride, ICl3 : It is an orange solid obtained by reacting I2 with excess of Cl2.
I2 + 3Cl2(excess) → 2ICl3
- This orange solid dimerises to form I2Cl6 having Cl — bridges.
Iodine chloride, ICl : It is obtained by reaction between equimolar amounts of I2 and Cl2. ∝-form of it is a ruby red solid While [β -form is a brown red solid.
I2 + Cl2 → 2ICl
Bromine pentafluoride, BrF5 : It is a colourless liquid and obtained by the reaction of bromine with the excess of fluorine.
Br2 + 5F2 (excess) → 2BrF5
Bromine chloride BrCl : It is a gas, obtained by the reaction of bromine with chlorine taken in equal volumes.
BI2 + Cl2 → 2BrCl
(ii) By the reaction of halogen with interhalogen compound :
Bromine fluoride (BrF) : It is a pale brown coloured gas prepared by the action of Br2 on BrF3.
Br2 + BrF3 → 3BrF
Bromine trifluoride (BrF3) : BrF3 can also be prepared by the action of Br2 on CIF3.
BI2 + CIF3 → 2BrF3 + BrCl
Special reaction for the preparation of [Cl] : ICl (Wij’s solution) can be prepared by the action of Iodine (I2) on potassium chlorate (KCIO3)
I2 + KCIO3 → ICl + KlO3 .
Some Properties of Interhalogen compounds :
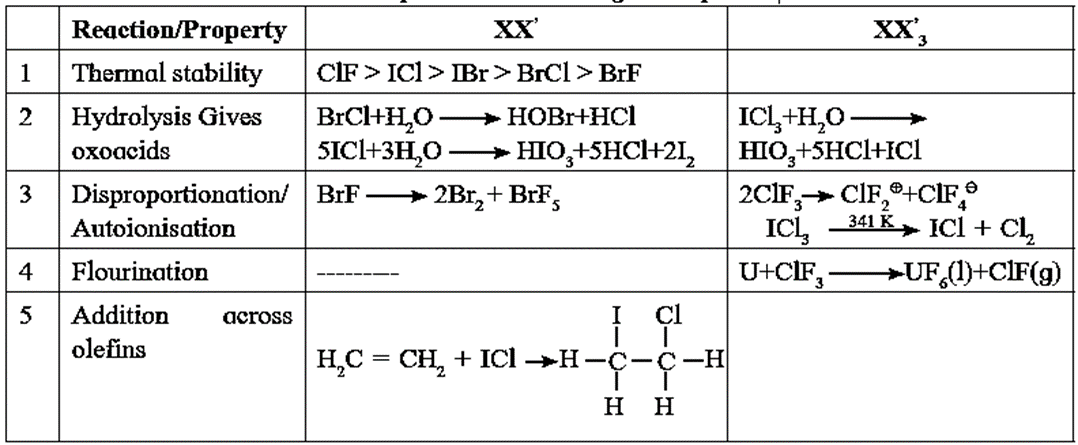
Q. Give one fluorinating reaction given by inter-halogen compounds.
Ans. Interhalogen compounds like CIF3, BrF3, etc. are very reactive and they are strong fluorinating agents.
U(s) + 3ClF3(l) → UF6(g) + 3ClF(g)
Uses of interhalogen compounds :
- ICl is used as a halogenating agent and also in the estimation of iodine number of fats and oils.
- In the preparation of polyhalides, interhalogen compounds are used.
- CIF3 and BrF3 are widely used as fluorinating agent. They are used in the separation of 235U isotope by fluorinating.
235U + 3ClF3(l) → 235UF6(g) + 3ClF(g)
- In propellants CIF3 and IF3 are used as oxidisers.
- They are used as non-aqueous solvents.
- The XX’ interhalogen compounds are used as a catalyst for the oxidation of As(III).
Structures of some Interhalogen Compounds :
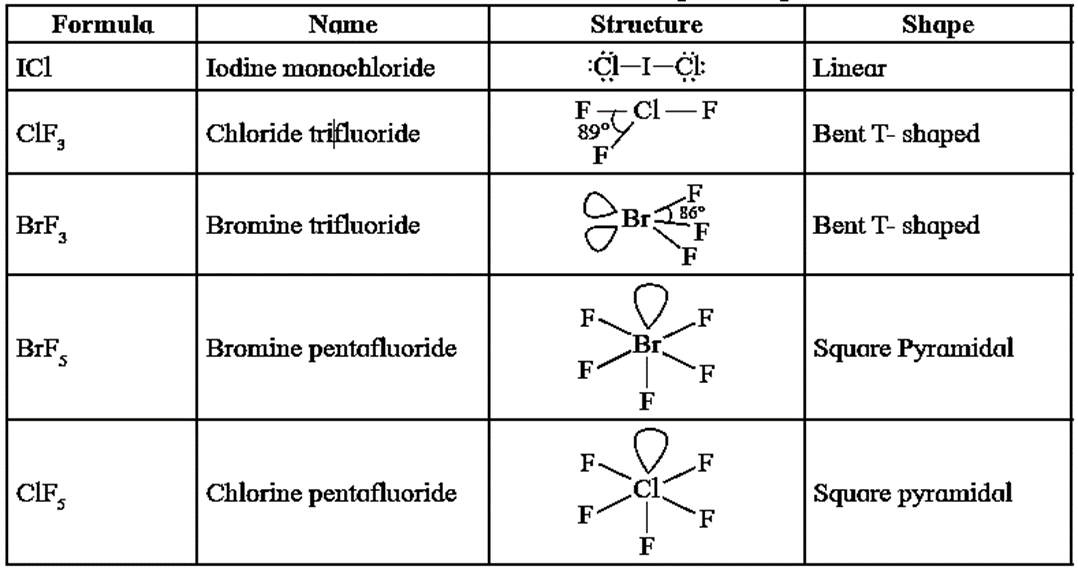
Oxidation states of central halogen atom in interhalogen compounds.
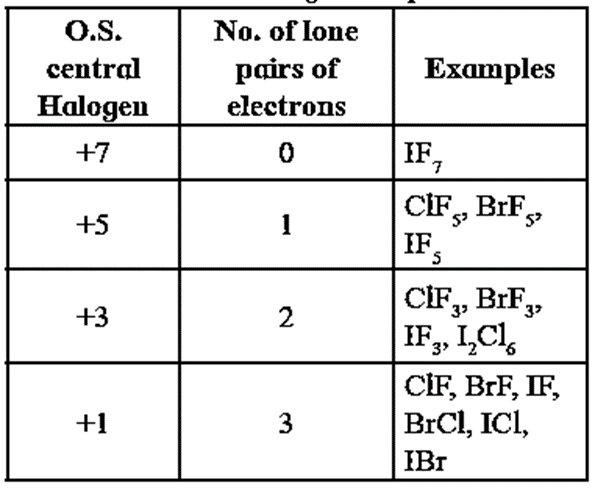
| Know This :
ICl in liquid state, undergoes auto ionization like water, to form a cation and an anion. 2ICl ⇌ I+ + ICl2− |
Compounds of Xenon :
Xenon fluorides :
Preparation of Xenon Fluorides : Xenon fluorides are generally prepared by direct reaction of xenon and fluorine in different ratios and conditions, such as temperature, electric discharge and photochemical reaction.
(i) Preparation of XeF2 :
Xe + F2 \(\frac{sealed\,\,Ni\,\,tube}{400^0C}\)-> XeF2
(ii) Preparation of XeF4 :
Xe + F2 \(\frac{Ni\,\,tube\,\,400^0C}{5-6\,atoms}\)-> XeF4
1 : 5
Xe + 2F2 \(\frac{electric\,\,discharge}{-80^0C}\)-> XeF4
(ii) Preparation of XeF6 :
Xe + 3F2 \(\frac{electric\,\,discharge}{low\,\,temp.}-->\) XeF6
Important chemical reactions of XeF2 :
(i) Hydrolysis : XeF2 undergoes hydrolysis to form HF
2XeF2 + 2H2O → 4HF + 2Xe + O2
(ii) Reaction with PF5 : XeF2 forms adducts on reaction with PF5.
XeF2 + PF5 → XeF2.PF5
Xenon trioxide :
Preparation of Xenon trioxide (XeO3) :
Fluorides of Xenon react with water to form XeO3.
3XeF4 + 6H2O → 2Xe + XeO3 + 12HF + O2
XeF6 + 3H2O → XeO3 + 6HF
Oxyfluorides of Xenon :
Xenon forms the following oxyfluorides : XeOF2, XeO2F2, XeO3F2, XeO2F4
Preparation :
(i) Partial hydrolysis of Xenon fluorides yields different oxyfluorides.
XeF4 + H2O \(\underrightarrow{80^0C}\) XeOF2 + 2HF
XeF6 + H2O → XeOF4 + 2HF
(ii) Reaction of Xenon oxyfluoride with SiO2 or hydrolysis yields xenon dioxydifluoride.
2XeOF4 + SiO2 →2XeO2F2 + SiF4
XeOF4 + H2O → XeO2F2 + 2HF
Structure of Xenon Compounds :
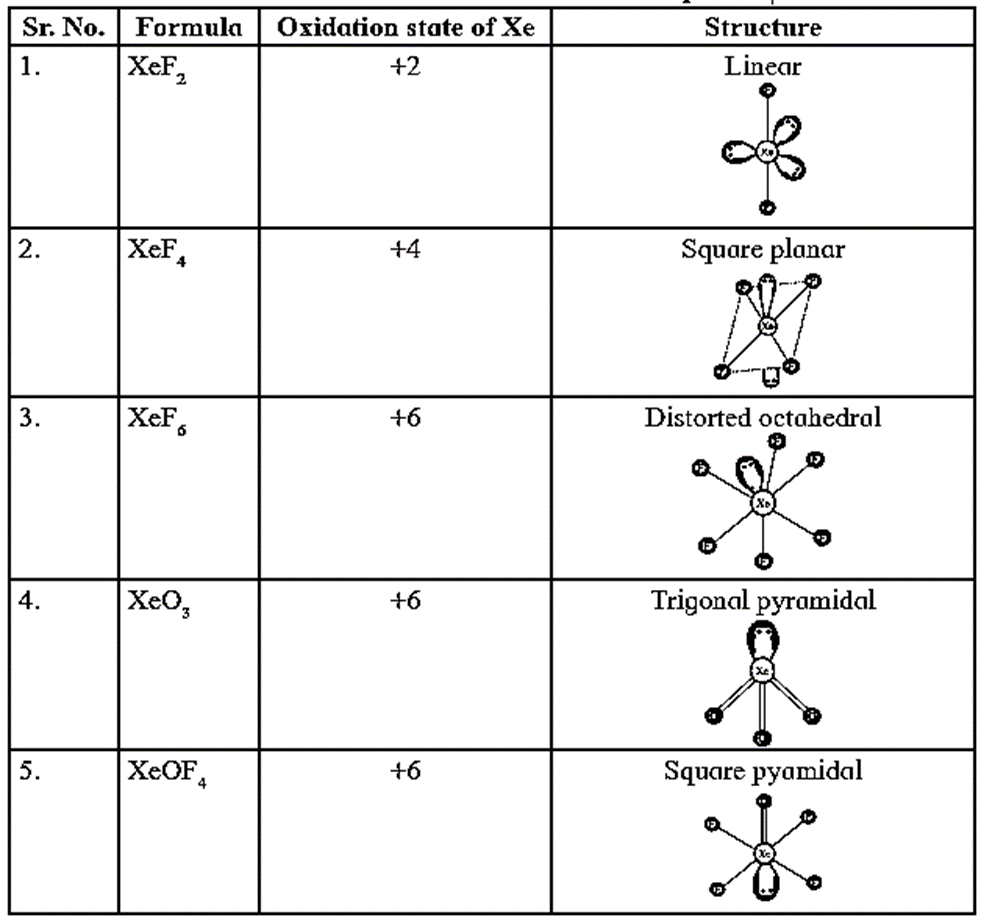
Remember :
- XeF2, XeF4, XeF6 are stable fluoride of xenon.
- Xenon also forms compounds with oxygen, such as XeO3, XeOF2, XeOF4, XeO2F2.
Uses of helium (He) :
- A mixture of helium (85%) and oxygen (15 %) is used for filling balloons.
- A mixture of helium and oxygen is also used for respiration by sea divers instead of air, because helium is less soluble in blood than nitrogen under high pressure. It is also used for treatment of asthma.
- Helium is used in producing inert atmosphere in metallurgical operations and welding of metals.
- Liquid helium is used in producing low temperature required for research.
- Helium is also used in producing lasers in low temperature gas thermometry.
- It is used in magnetic resonance imaging.
- It is used as a shielding gas for arc welding.
- It is used in supersonic wind tunnels.
- Helium nucleus is used as a bombarding particle for disintegration of atoms.
Uses of neon (Ne) :
- Neon is used in the production of neon discharge lamps and signs by filling Ne in glass discharge tubes.
- Neon signs are visible from a long distance and also have high penetrating power in mist or fog.
- A mixture of neon and helium is used in voltage stabilizers and current rectifiers.
- Neon is also used in the production of lasers and fluorescent tubes.
Uses of argon (Ar) :
- Argon is used to fill fluorescent tubes and radio valves.
- It is used to provide inert atmosphere for welding and production of steel.
- It is used along with neon in neon sign lamps to obtain different colours.
- A mixture of 85% Ar and 15% N2 is used in electric bulbs to enhance the life of the filament.
Chapter-7-Elements of Groups 16, 17, and 18-Text Book-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Notes-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Solution-PDF
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6-Chemical Kinetics – Online Notes
Next Chapter : Chapter-8-Transition and Inner transition Elements – Online Notes
We reply to valid query.