Electrochemistry
Maharashtra Board-Class-12-Chemistry-Chapter-5
Notes-Part-3
Topics to be Learn : Part-3
|
Reference electrodes :
A single electrode potential can’t be measured but the cell potential can be measured :
Explanation :
- The electrode potential, according to Nernst theory, is the potential difference between the metal and the ionic layer surrounding it at equilibrium, i.e. the potential across the electric double layer.
- For measuring the single electrode potential, one part of the double layer, that is metallic layer can be connected to the potentiometer but not the ionic layer. Hence, single electrode potential can’t be measured experimentally.
- When an electrochemical cell is developed by combining two half cells or electrodes, they can be connected to the potentiometer and the potential difference or cell potential can be measured.
Ecell : E2 − E1, where E1 and E2 are reduction potentials of two electrodes.
- If one of the electrode potentials is known or arbitrarily assumed and Ecell is measured by potentiometer, then potential of another electrode can be obtained. Therefore it is necessary to choose a reference electrode with arbitrarily fixed potential and measure the potentials of other electrodes.
- Therefore Standard Hydrogen Electrode (SHE) is selected assuming arbitrary potential 0.0 volt. Hence potentials of all other electrodes are referred to as hydrogen scale potentials.
Standard hydrogen electrode (SHE) :
A single electrode potential cannot be measured, but the cell potential can be measured experimentally. Hence, it is necessary to have a reference electrode. S.H.E. is a primary reference electrode.
Construction :
The standard hydrogen electrode (S.H.E.) consists of a glass tube at the end of which a piece of platinised platinum foil is attached as shown in below Fig. Around this plate there is an outer jacket of glass which has a side inlet through which pure and dry hydrogen gas is bubbled at one atmosphere pressure. The inner tube is filled with a little mercury and a copper wire is dipped into it. This provides an electrical contact with the platinum foil. The outer jacket ends into a broad opening.
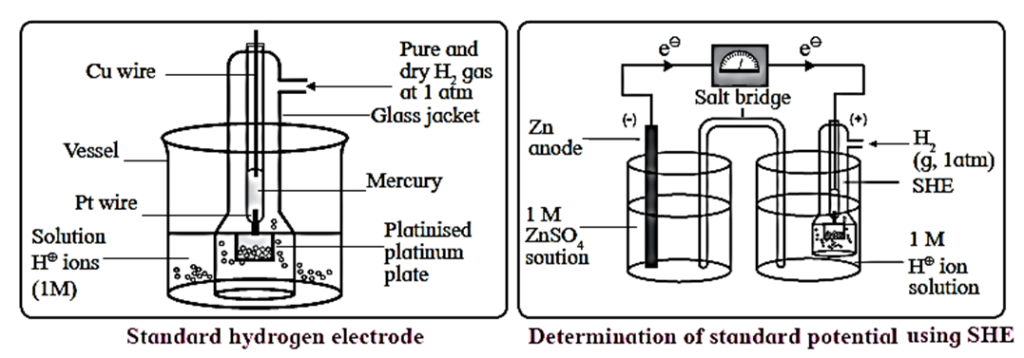
- The whole assembly is kept immersed in a solution containing hydrogen ions (H+) of unit activity.
- This electrode is arbitrarily assigned zero potential.
- The platinised platinum foil is used to provide an electrical contact for the electrode. This permits rapid establishment of the equilibrium between the hydrogen gas adsorbed by the metal and the hydrogen ions in solution.
Representation of S.H.E. :
H+|H2 (g, 1atm)| Pt
(1 M)
Working :
Reduction : H+(aq) + e− ⇌ ½ H2(g) E0 = 0.00 V
H2 gas in contact with H+(aq) ions attains an equilibrium establishing a potential.
Applications of SHE : A reversible galvanic cell with the experimental (indicator) electrode,
Zn2+|Zn(s) and SHE can be developed as follows
(1 M)
Zn |Zn2+|| H+(aq)|H2(g, 1 aim)|Pt
(1 M) (1 M)
Then in general,
Ecell = ESHE − EM
= 0 − EM
= − EM
Thus the potential can be directly obtained.
Disadvantages (Drawbacks or Difficulties) :
- It is difficult to construct and handle SHE.
- Pure and dry H2 gas cannot be obtained.
- Pressure of H2 gas cannot be maintained exactly at 1 atmosphere.
- The active mass or concentration of H+ from HCl cannot be maintained exactly unity.
Potential of hydrogen electrode:
Hydrogen gas electrode is represented as,
H+(aq)|H2 (g, 1atm)| Pt
Electrode reduction reaction is,
2 H+(aq) + 2 e− —> H2(g)
By Nernst equation, the reduction potential is,
EH2 = \(E^0_{H_2}-\frac{0.0592}{2}log_{10}\frac{P_{H_2}}{[H^+]^2}\)
‘.’ E0H2 = E0SHE = 0.0 V
∴ EH2 = \(-\frac{0.0592}{2}log_{10}\frac{P_{H_2}}{[H^+]^2}\)
If H2 gas is passed at 1 atm, then PH2 = 1 atm
∴ EH2 = \(-\frac{0.0592}{2}log_{10}\frac{1}{[H^+]^2}\)
= \(-\frac{0.0592}{2}log_{10}[H^+]^2\)
= −0.0592 log10[H+]
= 0.0592[− log10(H+)]
= 0.0592 pH
∴ pH = EH2/0.0592
Galvanic cells useful in day-to-day life :
Voltaic (or galvanic) cells in common use can be classified as primary and secondary cells.
Primary voltaic cells :
- When a galvanic cell discharges during current generation, the chemicals are consumed.
- In primary voltaic cell, once the chemicals are completely consumed, cell reaction stops.
- The cell reaction cannot be reversed even after reversing the direction of current flow or these cells cannot be recharged.
- The most familiar example is dry cell.
Secondary voltaic cells :
- These are the voltaic cells in which the electrical energy or cell potentials are not developed within the cell but electrical energy can be stored or cell potentials can be regenerated by passing electricity from the external source of electricity.
- Since the electrical energy obtained is second hand, these cells are called secondary cells or accumulators or storage cells.
- These cells can be recharged by passing electric current in opposite direction from the external source of higher emf.
- Therefore the secondary cells are reversible cells.
- For example, lead accumulator (lead storage battery).
Dry cell (Leclanche' cell) :
- Leclanche’s cell is a primary voltaic cell.
- It contains moist viscose aqueous paste of the electrolytes rather than mobile liquid electrolyte.
- It is a non-rechargeable irreversible voltaic cell.
Construction :
- It consists of a small zinc vessel which serves as an anode (negative electrode).
- The zinc vessel contains a porous paper bag containing an inert graphite (C) electrode which serves as cathode, immersed in a paste of MnO2 and carbon black.
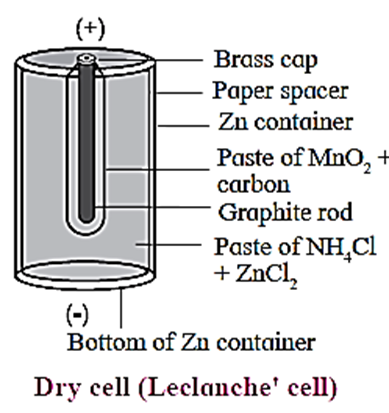
- This paper bag divides the dry cell into two compartments, namely anode and cathode compartments.
- The rest of the cell is filled with a moist paste of NH4Cl and ZnCl2 which acts as an electrolyte for zinc anode.
- The graphite rod is fitted with a metal cap and the cell is sealed to prevent the drying of moist paste by evaporation.
The dry cell can be represented as,
Zn|ZnCl2(aq), NH4Cl(aq), MnO2(s)|C+.
Reactions in the dry cell :
(i) Oxidation at zinc anode :
Zn(s) → Zn2+(aq) + 2e− (oxidation half reaction)
(ii) Reduction at graphite (C) cathode :
The electrons released in the oxidation reaction at anode, flow to cathode through external circuit.
Hydrogen in NH4+ ion is reduced to molecular hydrogen which reduces MnO2 to Mn2O3.
2NH4+(aq) + 2e− → 2NH3(aq) + H2(g)
2MNO2(s) + H2(g) → Mn2O3(s) + H2O(l)
The overall cell reaction is
Zn(S) + 2NH4+(aq) + 2MnO2(S) → Zn2+(aq) + 2NH4+(aq) + Mn2O3(s) + H2O(l)
The average cell potential of dry cell is 1.5 V.
(iii) Zn2+ react with NH3 and form a complex.
Zn2+(aq) + 4NH3(aq) → [Zn(NH3)4] 2+(aq)
Since Zn2+ ions are removed, the overall cell reaction can’t be reversed.
Uses of dry cell : Dry cell is used as a source of power in flashlights, portable radios, tape recorders, clocks and so forth. Since they are available in small size and portable, they can be used conveniently.
| Know This :
Alkaline dry cell : The Leclanche' dry cell works under acidic conditions due to the presence of NH4Cl.The difficulty with this dry cell is that Zn anode corrodes due to its actions with H+ ions from NH4+ ions. This results in shortening the life of dry cell. To avoid this a modified or of the dry cell called alkaline dry cell has been proposed. In alkaline dry cell NaOH or KOH is used as electrolyte in place of NH4Cl. The alkaline dry cell has longer life than acidic dry cell since the Zn corrodes more slowly. |
Lead storage battery (Lead accumulator) :
- Because electrical energy and emf are not generated within the cell, but are previously stored by passing an electric current, the lead accumulator is a secondary electrochemical cell. As a result, it is also known as a lead accumulator or a lead storage battery.
- It is reversible since the electrochemical reaction can be reversed by passing an electric current in opposite direction and consumed reactants can be regenerated.
- Hence battery can be charged after it is discharged.
Construction : In a lead accumulator, the negative terminal (anode) is made up of lead sheets packed with spongy lead, while the positive terminal (cathode) is made up of lead grids packed with PbO2.
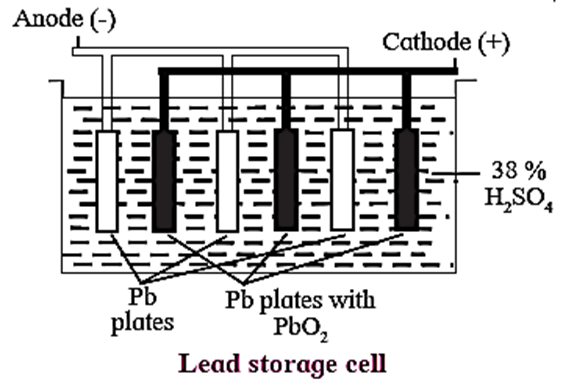
Sulphuric acid of about 38% strength (% w/w) or specific gravity 1.28 or 4.963 molar is the electrolyte in which the lead sheets and lead grids are dipped.
The positive terminal and negative terminal are alternatively arranged in the electrolyte and are separately interconnected.
Notation of the cell : The cell is formulated as Pb(s)|PbSO4(s)|38%H2SO4(aq)|PbSO4(s)|PbO2(s)|Pb(s)
Working of a lead accumulator :
Discharging : When the electric current is withdrawn from lead accumulator, the following reactions take place :
Oxidation at the — ve electrode or anode :
Pb(s) → Pb2+(aq) + 2e− (oxidation)
Pb2+(aq) + SO42−(aq) → PbSO4(s) (precipitation)
----------------------------------------
Pb(s) + SO42−(aq) → PbSO4(s) + 2e− (overall oxidation)
Reduction at cathode (+) : The electrons produced at anode travel through external circuit and re-enter the cell at cathode. At cathode PbO2 is reduced to Pb2+ ions in presence of H+ ions. Subsequently Pb2+ ions so formed combine with SO42− ions from H2SO4 to form insoluble PbSO4 that gets coated on the electrode.
PbO2(s) + 4H+(aq) + 2e− → Pb2+(aq) + 2H2O(l) (reduction)
Pb2+(aq) + SO42−(aq) → PbSO4(s) (precipitation)
--------------------------------------------------------------
PbO2(s) + 4H+(aq) + SO42−(aq) + 2e−→ PbSO4(s) + 2H2O(l) (overall reduction)
Net cell reaction during discharge: The net cell reaction is the sum of overall oxidation at anode and overall reduction at cathode.
Pb(s) + PbO2(s) + 4H+(aq) + 2SO42−(aq) → 2 PbSO4(s) + 2H2O(l)
or
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l) ...(iii)
The cell potential or emf of the cell depends upon the concentration of sulphuric acid. During the operation, the acid is consumed and its concentration decreases and specific gravity decreases from 1.28 to 1.17. As a result, the emf of the cell decreases. The emf of a fully charged cell is about 2.0 V.
Recharging of the cell : When the discharged battery is connected to external electric source and a higher external potential is applied the cell reaction gets reversed generating H2SO4.
Reduction at the − ve electrode or cathode :
PbSO4(s) + 2e− → Pb(s) + SO42−(aq)
Oxidation at the +ve electrode or anode :
PbSO4(s) + 2H2O(l) → PbO2(s) + 4H+(aq) + SO42−(aq) + 2e−
The net reaction during charging is
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 4H+(aq) + 2SO42−(aq)
OR
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H2SO4(aq)
The emf of the accumulator depends only on the concentration of H2SO4.
Applications :
- It is used as a source of d.c. electric supply.
- It is used in automobile in ignition circuits and lighting the head lights by connecting 6 batteries giving 12 V potential.
- It is also used in invertors.
Mercury battery : Mercury battery is a secondary dry cell and can be recharged.
The mercury battery consists of zinc anode, amalgamated with mercury. The cathode is a paste of Hg and carbon. The electrolyte is strongly alkaline and made of a paste of KOH and ZnO.
The electrode ractions and net cell reaction are :
Zn(Hg)+2OH−(aq) → ZnO(s) + H2O(l) + 2e− (anode oxidation)
HgO(s)+ H2O(l)+2e− → Hg(l) + 2OH−(aq) (cathode reduction)
-----------------------------------------------------
Zn(Hg) + HgO(s) → ZnO(s) + Hg(l) (overall cell reaction)
- The overall reaction involves only solid substances.
- There is no change in electrolyte composition during operation.
- The mercury battery, therefore, provides more constant voltage (1.35V) than the Leclanche' dry cell.
- It also has considerably higher capacity and longer life than dry cell.
Applications :
The mercury dry cell finds use in hearing aids, electric watches, pacemakers, etc.
Nickel-Cadminum (NICAD) cell :
- It is a secondary dry cell.
- It is rechargable, hence it is a reversible cell.
- It consists of a cadmium electrode in contact with an alkali and acts as anode while nickel (IV) oxide, NiO2 in contact with an alkali acts as cathode. The alkali used is moist paste of KOH.
Reactions in the cell :
(i) Oxidation at cadmium anode :
Cd(s) + 2OH−(aq) → Cd(OH)2(s) + 2e−
(ii) Reduction at NiO2(s) cathode :
NiO2(s) + H2O(l) + 2e− → Ni(OH)2(s) + 2OH−(aq)
The overall cell reaction is the combination of above two reactions.
Cd(s) + NiO2(s) + 2 H2O(l) → Cd(OH)2(s) + Ni(OH)2(s)
- Since the net cell reaction doesn’t involve any electrolytes but solids, the voltage is independent of the concentration of alkali electrolyte.
- The cell potential is about 1.4 V.
- This cell has longer life than other dry cells.
Fuel cells :
Principle :
- The operation of fuel cells is based on the fact that combustion reactions are redox in nature and can generate electricity.
- The fuel cells differ from ordinary galvanic cells in that the reactants are not placed within the cell. They are continuously supplied to electrodes from a reservoir.
- In these cells one of the reactants is a fuel such as hydrogen gas or methanol. The other reactant such as oxygen, is oxidant.
- The simplest fuel cell is hydrogen-oxygen fuel cell.
Hydrogen-oxygen fuel cell : In H2-O2 fuel cell, the fuel is hydrogen gas. Oxygen gas is an oxidising agent. The energy of the combustion of hydrogen is converted into electrical energy.
Construction :
In fuel cell the anode and cathode are porous electrodes with suitable catalyst like finely divided platinum.
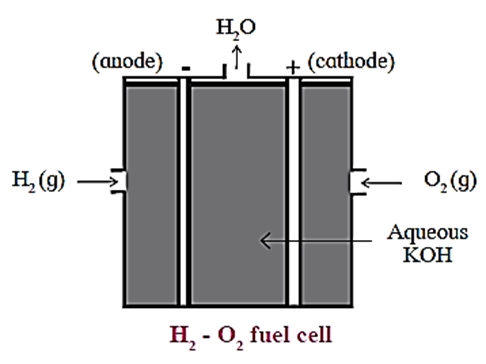
The electrolyte used is hot aqueous KOH solution in which porous anode and cathode carbon rods are immersed.
H2 is continuously bubbled through anode while O2 gas is bubbled through cathode.
Working (cell reactions) :
Oxidation at anode : At anode, hydrogen gas is oxidised to H2O.
2H2(g) + 4OH−(aq) → 4H2O(l) + 4e− (oxidation half reactionn)
Reduction at cathode : The electrons released at anode travel to cathode through external circuit and reduce oxygen gas to OH−.
O2(g) + 2H2O(l) + 4e− → 4OH−(aq) (reduction half reaction)
Net cell reaction : Addition of both the above reactions at anode and cathode gives a net cell reaction.
2H2(g) + O2(g) → 2H2O(l) (overall cell reaction)
Representation of the cell :
Pt |H2(g)|NaOH(aq)|O2(g)| Pt+
(hot)
E0cell = E0cathode − E0anode
= 0.4 − (− 0.83)
= 1.23 V
The overall cell reaction is an exothermic combustion reaction. However in this, H2 and O2 gases do not react directly but react through electrode reactions. Hence the chemical energy released in the formation of O-H bonds in H2O, is directly converted into electrical energy.
Advantages :
- The fuel cell operates continuously as long as H2 and O2 gases are supplied to the electrodes.
- The cell reactions do not cause any pollution.
- The efficiency of this galvanic cell is the highest about 70 % as compared to ordinary galvanic cells.
Drawbacks of H2-O2 fuel cell :
- The cell requires expensive electrodes like Pt, Pd.
- In practice, voltage is less than 1.23 volt due to spontaneous reactions at the electrodes.
- H2 gas is expensive and hazardous.
Applications of fuel cells
- The fuel cells are used on experimental basis in automobiles.
- The fuel cell are used for electrical power in the space programme.
- In space crafts the fuel cell is operated at such a high temperature that the water evaporates at the same rate as it is formed. The vapour is condensed and pure water formed is used for drinking by astronauts.
- In future, fuel cells can possibly be explored as power generators in hospitals, hotels and homes.
Similarity and Difference in fuel cells and galvanic cells :
Similarity :
- In both the cells, there is oxidation at anode and reduction at cathode.
- The cell potential is developed due to net redox reactions.
- Both are galvanic cells.
Difference in fuel cells and galvanic cells :
| Fuel cells | Galvanic cells |
| Fuel cells involve electrodes with large surface area. | Galvanic cells involve electrodes with compact surface area. |
| Involve gaseous materials on a large scale | Involve gaseous materials at a definite pressures along with electrolytes or there may not be gases. |
| Cell potential is developed due to exothermic combustion reactions. | Cell potential is developed due to normal redox reactions. |
| Gaseous electrode materials are continuously supplied from outside | Electrode materials have constant concentration or may change due to reactions. |
| Cannot recharge | It can recharge. |
Electrochemical series (Electromotive series) :
Electrochemical series (Electromotive series) : It is defined as the arrangement in a series of electrodes of elements (metal or non-metal in contact with their ions) with the electrode half reactions in the decreasing order of their standard reduction potentials. .
Key points of electrochemical series :
The conventions used in the construction of electrochemical series (or electromotive series) are as follows :
- The (reduction) electrodes or half cells of the elements are written on the left hand side of the series and they are arranged in the decreasing order of their standard reduction potentials (E0red).
- Reduction half reactions are written for each half cell in such a way that the species with higher oxidation state and electrons are on left hand side while reduced species with lower oxidation state are on right hand side.
- The standard reduction potential of standard hydrogen electrode is 0.00 V, i.e., E°H2 = 0.0 V.
- The electrodes and half cell reactions with positive E0red values are located above hydrogen and those with negative E0red values below hydrogen. Above hydrogen, positive E0red values increase, while below hydrogen negative E0 values increase.
- The positive E0red values indicate the tendency for reduction and the negative E0red values indicate the tendency for oxidation.
- The elements, whose electrodes are at the top of the series having high positive values for E0red are good oxidising agents.
- The elements, whose electrodes are at the bottom of the series having high negative values for E0red are good reducing agents.
Applications of electrochemical series :
(i) Predicting relative strength of oxidising agents :
Relative strength of oxidising agents in terms of E0red values : The E0red value is a measure of the tendency of the species to be reduced i.e., to accept electrons and act as an oxidising agent. The species mentioned on left hand side of the half reactions are oxidising agents.
The substances in the upper positions in the series and hence in the upper left side of the half reactions have large positive E0red values hence are stronger oxidising agents. For example, F2, Ce4+, Au3+, etc. As we move down the series, the oxidising power decreases. Hence from the position of the elements in the electrochemical series, oxidising agents can be selected.
(ii) Predicting relative strength of reducing agents :
Relative strength of reducing agents in terms of E0red values : The lower E0red value means lower tendency to accept electrons but higher tendency to lose electrons. The tendency for reverse reaction or oxidation increases as E0red becomes more negative and we move towards the lower side of the series. For example, Li, K, Al, etc. are good reducing agents.
(iii) Identifying the spontaneous direction of a reaction :
From the standard reduction potentials, E0red, the spontaneity of a redox reaction can be determined. The difference between E0red values for any two electrodes represents cell potential E0cell constituted by them.
If E0cell is positive then the reaction is spontaneous while if E0cell is negative the reaction is non-spontaneous. For example, E0Mg and E°Ag have values — 2.37 V and 0.8 V respectively.
Then Mg will be a better reducing agent than Ag.
Therefore Mg can reduce Ag+ to Ag.
The corresponding reaction will be,
Mg(s) + 2Ag+(aq) → Mg2+(aq) + 2Ag(s)
E0cell = E0Ag − E0Mg = 0.8 − (− 2.37) = 3.17 V
Therefore above reaction in the forward direction will be spontaneous while in the reverse direction will be non-spontaneous since for it E0cell = − 3.17 V,
(iv) To calculate the standard cell potential E0cell.
From the electrochemical series, the standard cell potential, E0cell from the E0red values for the half reactions given can be calculated.
For example,
Zn2+ + 2e− → Zn(s) E0Zn = −0.76 V
Cu2+ + 2e− → Cu (s) E0Cu = −0.34 V
For the cell,
Zn | Zn2+(aq) (1 M) || Cu2+(aq) (1 M) |Cu
E0cell = E0Cu − E0Zn = 0.34 − (− 0.76) = 1.1 V.
General rules
- An oxidizing agent can oxidize any reducing agent that appears below it, and cannot oxidize the reducing agent appearing above it in the electrochemical series.
- An reducing agent can reduce the oxidising agent located above it in the electrochemical series.
Remember :
|
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-4- Chemical Thermodynamics – Online Notes
Next Chapter : Chapter-6-Chemical Kinetics – Online Notes
We reply to valid query.