Aldehydes, Ketones and Carboxylic acids
Maharashtra Board-Class-12-Chemistry-Chapter-12
Notes-Part-3
Topics to be Learn : Part-3
|
Polarity of carbonyl group :
The polarity of a carbonyl group is due to higher electronegativity of oxygen compared to carbon. It is explained on the basis of resonance involving a neutral and dipolar structures.
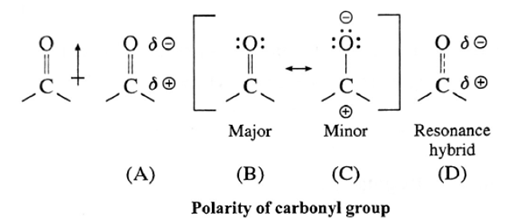
The carbonyl carbon has positive polarity [see structures (A) and (D)]. Therefore, it is electron deficient. As a result, this carbon atom is electrophilic (electron loving) and is susceptible to attack by a nucleophile Nu : —).
Reactivity of aldehydes and ketones :
The reactivity of aldehydes and ketones is due to the polarity of carbonyl group which results in electrophilicity of carbon. The reactivity is further explained on the basis of electronic effect and steric effects.
(a) Influence of electronic effects : A ketone has two electron donating alkyl groups (+I effect) bonded to carbonyl carbon which are responsible for decreasing its positive polarity and electrophilicity. In contrast, aldehydes have
only electron donating group bonded to carbonyl carbon. This shows aldehydes are more electrophilic than ketones.
(b) Steric effects : Two bulky alkyl groups in ketone come in the way of incoming nucleophile. This is called steric hindrance to nucleophilic attack.
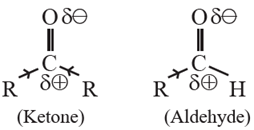
On the other hand, nucleophile can easily attack the carbonyl carbon in aldehyde because it has one alkyl group and is less crowded or sterically less hindered. Hence aldehyde are more reactive and can easily attacked by nucleophiles.
| Remember :
Aromatic aldehydes are less reactive than aliphatic aldehydes in nucleophilic addition reactions. This is due to electron-donating resonance effect of aromatic ring which makes carbonyl carbon less electrophilic. |
Commercially available forms of formaldehyde and acetaldehyde:
- Formaldehyde is available commercially as solid polymer called paraformaldehyde HO-[CH2 – O-]n H and trioxane (CH2O)3 (Trioxane has cyclic structure). These are convenient for use in chemical reactions as source of formaldehyde.
- Aqueous solution of formaldehyde gas is called formalin, which is used for preservation of biological and anatomical specimens.
- When dry formaldehyde is required, it is obtained by heating araformaldehyde or trioxane.
- Acetaldehyde is also conveniently used as solid trimer (paraldehyde) and tetramer (metaldehyde).
Chemical properties of aldehydes and ketones :
Laboratory tests for aldehydes and ketones :
· Aldehydes are easily oxidized to carboxylic acids and therefore, act as reducing agents toward mild oxidizing agents.
- Ketones, do not have hydrogen atom directly attached to carbonyl carbon. Hence, they are not oxidized by mild oxidizing agents.
- On the basis of this difference in the reactivity, aldehydes and ketones are distinguished by tests.
Tests given by only aldehydes :
(i) Schiff test : When alcoholic solution of aldehyde is treated with few drops of Schiff 's reagent, pink or red or magenta colour appears. This confirms the presence of aldehydic (-CHO) group.
Schiff’s reagent :
- Schiff’s reagent is prepared by dissolving pink p-rosaniline hydrochloride (dye Fuchsin) in water and passing SO2 gas till the pink solution is decolourised.
- Schiff’s reagent is an oxidising agent.
- When an aldehyde is added to Schiff’s reagent, the colourless solution turns pink or in magenta colour and aldehyde is oxidised to a carboxylic acid.
- This test is not given by ketones, hence, used to distinguish between aldehyde and ketone.
(ii) Tollens' test or silver mirror test :
- When an aldehyde is boiled with Tollens' reagent (ammonical silver nitrate), silver mirror is formed. The aldehyde is oxidized to carboxylate ion by Tollens' reagent and Ag+ ion is reduced to Ag.
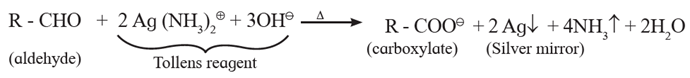
- This test is not given by ketones.
- Hence Tollen’s reagent is used to distinguish between aldehydes and ketones.
Tollen’s reagent :
- Tollen’s reagent is an ammoniacal silver nitrate, [Ag(NH3)2]+ OH—
- lt is prepared by adding NH,OH solution to silver nitrate solution.
AgNO3 + 3NH4OH → [Ag(NH3)2]+ OH— + NH4NO3 + 2H2
- It is a stronger oxidising agent than Fehling solution. Aldehyde when heated with Tollen’s reagent, silver mirror is deposited.
(iii) Fehling’s solution test :
- Fehling’s solution is a complex of cupric ions with tartaric acid.
- It is a mild oxidising agent.
- Fehling’s solution is a mixture of Fehling’s solution ‘A’ containing CuSO4 solution and Fehling’s solution ‘B’ containing sodium potassium tartarate (Rochella salt) in caustic soda (NaOH) solution.
- When an aldehyde is heated with Fehling’s solution, the deep blue colour of the solution disappears and Cu+2 (cupric ion) is reduced to Cu+ ion a red precipitate of cuprous oxide, Cu2O is obtained while aldehyde is oxidized to a carboxylate ion.
Example :
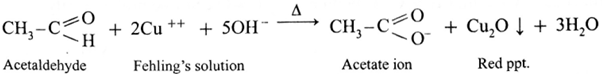
- This test is not given by ketones, since they cannot be oxidised by Fehling solution.
- Aromatic aldehydes are not oxidised by Fehling solution.
- Hence this test is used to distinguish between aldehydes and ketones.
Examples :
Action of Fehling’s solution on ethanal :
- When ethanal is heated with Fehling’s solution, the deep blue colour of the solution disappears and a red precipitate of Cu2O is obtained.
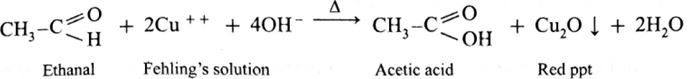
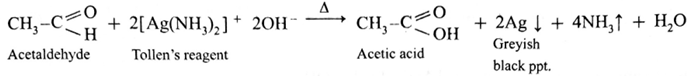
Action of Tollen’s reagent or Ammonical silver nitrate on ethanal :
- When ethanal is heated with Tollen’s reagent a greyish black precipitate or deposition of silver is obtained.
Laboratory test for ketonic group or sodium nitroprusside test :
- When a freshly prepared sodium nitroprusside solution is added to a ketone, mixture is shaken well and basified by adding sodium hydroxide solution drop by drop, red colour appears in the solution, which indicates the presence of ketonic (>C = O) group.
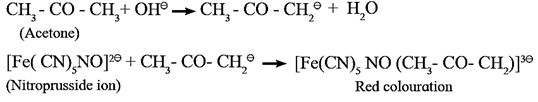
- The anion of ketone formed by alkali reacts with nitroprusside ion to form a red coloured complex which indicates the presence of ketonic group.
Chemical reactions of aldehydes and ketones with nucleophile :
In all these reactions the nucleophilic reagent brings about reactions by attacking on positively polarized electrophilic carbonyl carbon in aldehydes and ketones.
(1) Addition of hydrogen cyanide (H-CN) :
Hydrogen cyanide (weak acid) adds across the carbon-oxygen double bond in aldehydes and ketones to produce compounds called cyanohydrins. The negative part of the reagent ( CN) attacks the electrophilic carbon of carbonyl group. The reaction requires either acid or base as catalyst.
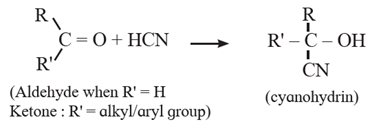
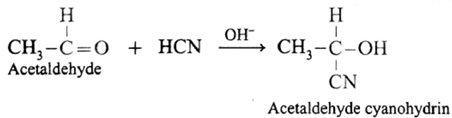
(i) Action of HCN on acetaldehyde : When acetaldehyde is treated with hydrogen cyanide, acetaldehyde cyanohydrin is formed.
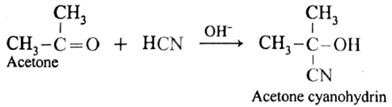
(ii) Action of HCN on acetone : When acetone is treated with hydrogen cyanide, acetone cyanohydrin is formed.
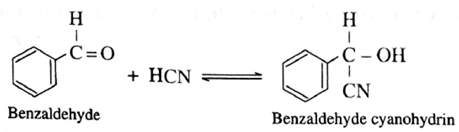
(iii) Action of HCN on benzaldehyde : When benzaldehyde is treated with hydrogen cyanide, benzaldehyde cyanohydrin is formed.
Remember :
- Cyanohydrin formation is a 'step-up' reaction as a new carbon- carbon single bond is formed.
- The - C ≡ N group can be converted to –COOH, - CH2 - NH2 and so on.
- Therefore, cyanohydrins are used as intermediate in step up synthesis.
(2) Addition of NaHSO3 (Sodium bisulphite) :
- Aldehydes and ketones react with saturated aqueous solution of sodium bisulfite to give crystalline precipitate of sodium bisulfite adduct (addition compound).
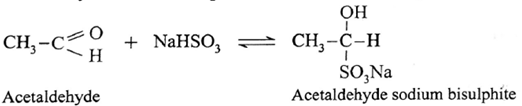
(i) Action of sodium bisulphite on Acetaldehyde :
Acetaldehyde reacts with saturated aqueous solution of sodium bisulphite (NaHSO;) and forms crystalline acetaldehyde sodium bisulphite. It is water soluble crystalline solid.
(ii) Action of sodium bisulphite on Acetone (propanone) :
Acetone reacts with saturated aqueous solution of sodium bisulphite (NaHSO3) and forms crystalline acetone sodium bisulphite. It is water soluble crystalline solid.
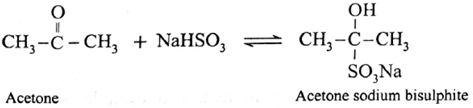
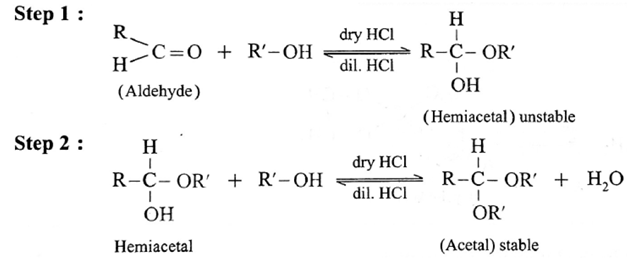
(3) Addition of alcohols :
Aldehyde reacts with one molecule of anhydrous monohydric alcohol in presence of dry hydrogen chloride to give alkoxyalcohol known as hemiacetal, which further reacts with one more molecule of anhydrous monohydric alcohol to give a geminaldialkoxy compound known as acetal as shown in the reaction.
Ketones react with 1, 2— or 1, 3— diols in presence of dry hydrogen chloride to give five or six-membered cyclic ketals.
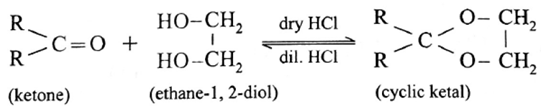
(i) Action of ethanol on acetaldehyde :
Acetaldehyde reacts with one equivalent of monohydric alcohol in the presence of dry hydrogen chloride to form an intermediate known as hemiacetal, which further adds another molecule of alcohol to form a gem-dialkoxy compound known as acetal.

(ii) Action of ethylene glycol on acetone :
Acetone reacts with ethylene glycol under similar conditions to form cyclic products known as ethylene glycol ketals.
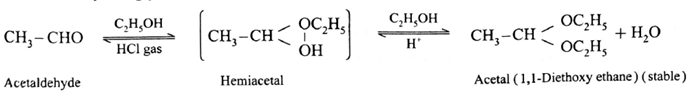
Know This :
|
(4) Addition of Grignard reagent :
The carbonyl compounds like aldehydes and ketones react with Grignard reagent (R-Mg-X) in dry ether and form a complex which on further hydrolysis with acid forms the corresponding alcohol.
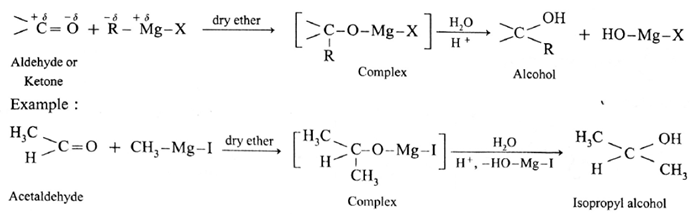
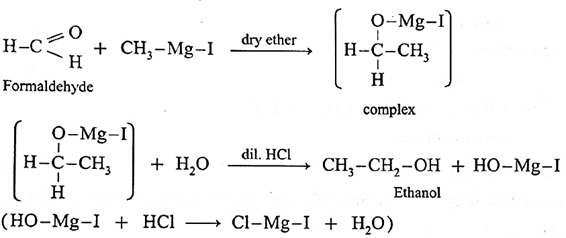
Action of Grignard reagent, CH3-Mg-I on formaldehyde :
Grignard reagent with formaldehyde gives a primary alcohol.
Formaldehyde on reaction with Grignard reagent, CH3-Mg-I in dry ether forms a complex which on hydrolysis with dilute HCI forms ethyl alcohol.
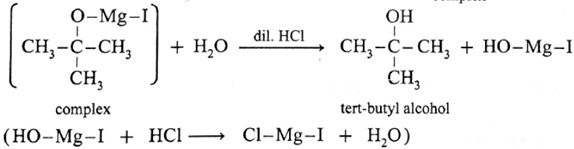
Action of Grignard reagent, CH3-Mg-I on acetone :
Acetone on reaction with Grignard reagent, CH3-Mg-I in dry ether forms a complex which on hydrolysis with dilute HCI forms tert-butyl alcohol.
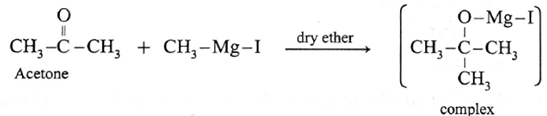
(5) Nucleophilic addition –elimination of aldehydes and ketones with ammonia derivatives :
Aldehydes and ketones undergo addition elimination with some ammonia derivatives (NH2-Z ) to give product containing C = N bonds (imines). The reaction is reversible and takes place in weakly acidic medium. The substituted imine is called a Schiff 's base. Schiff bases are solids and have sharp melting points.
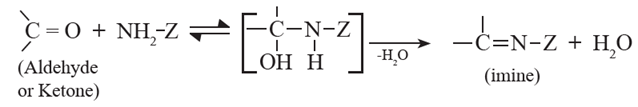
Various ammonia derivatives
Z = alkyl, - NH2, OH, -NH6H5, -NHCONH2 , -NHC6H3(NO2)2
Examples :
(i) Action of ethylamine on acetaldehyde :
Acetaldehyde on reaction with ethyl amine forms imine (Schiff base).
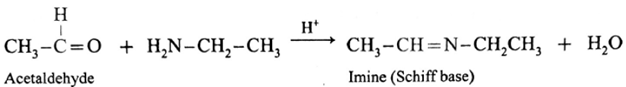
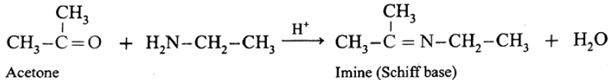
(ii) Action of ethylamine on acetone :
Acetone on reaction with ethyl amine forms imine (Schiff base).
Nucleophilic addition – elimination reactions of aldehydes and ketones with ammonia derivatives :

- All aldehydes and ketones give similar reactions.
- The resulting products have high molecular mass and are crystalline solids.
- These reactions are, therefore, useful for characterization of the original aldehydes and ketones.
Remember : In strong acidic medium, nitrogen atom of ammonia derivative H2N-Z is protonated to form (H3N+ – Z) ion which is no longer a nucleophile.
(6) Haloform reaction :
ketone containing —COCH3 group is oxidised by sodium hypohalite a mixture of (sodium hydroxide and halogen) results in the formation of sodium salt of carboxylic acid having one carbon atom less than that of ketone and methyl group is converted to haloform.
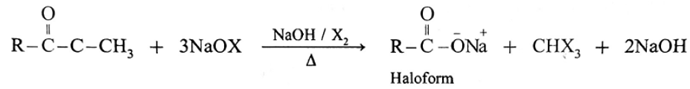
Acetaldehyde is the only aldehyde which gives haloform reaction. In this reaction, R may be hydrogen, methyl group or aryl group and X may be Cl, Br or I. The reaction is given by all methyl ketones (CH3—CO-R) and all alcohols containing CH3—(CHOH) group.
When a methyl ketone is warmed with iodine and sodium hydroxide, a yellow precipitate of iodoform is obtained. The iodoform reaction is used as a qualitative test for detection of CH3CO- group in a organic compound.
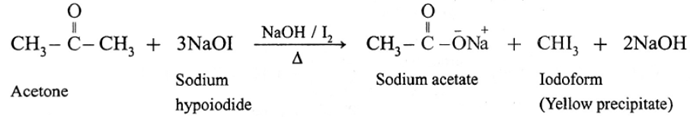
For an iodoform test, the carbonyl compound must have CH3CO- group
The compounds that give positive iodoform test :
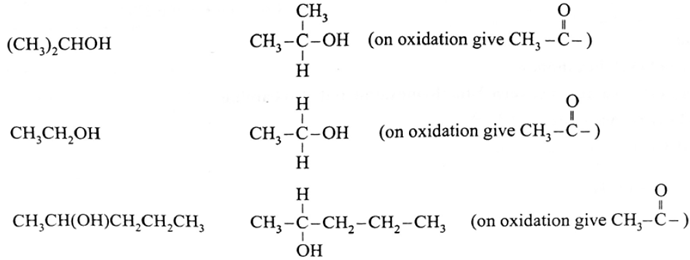
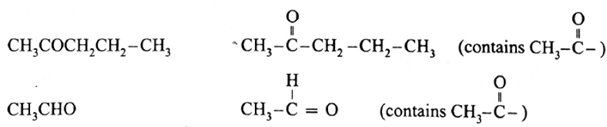
Remember :
- If C=C bond is present in a given aldehyde or ketone or methyl ketone, it is not attacked by hypohalite.
- Non methyl ketones do not give a positive iodoform test.
- Secondary alcohols having CH3-CHOH- group give positive iodoform test because the reagent first oxidizes it to a CH3-CO- group which subsequently forms iodoform.
(7) Aldol condensation :
- The carbon atom adjacent to carbonyl carbon atom is called a-carbon atom (α—C) and the hydrogen atom attached to a-carbon atom is called α-hydrogen atom (α—H).
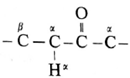
- The α-hydrogen of aldehydes and ketones is acidic in nature due to (i) the strong—I effect of carbonyl group (ii) resonance stabilization of the carbanion.
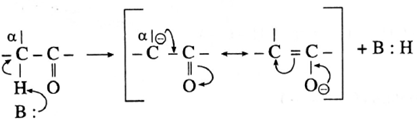
- Aldol condensation reaction is characteristic reaction of aldehydes and ketones containing active a-hydrogen atom.
- ‘When aldehydes or ketones containing α —H atoms are warmed with a dilute base or dilute acid, two molecules of them undergo self condensation to give f-hydroxy aldehyde (aldol) or f-hydroxy ketone (ketol) respectively. The reaction is known as Aldol addition Reaction.
- In aldol condensation, the product is formed by the nucleophilic addition of s-carbon atom of a second molecule which gets attached to carbonyl carbon atom of the first molecule and α -hydrogen atom of the second molecule gets attached to carbonyl oxygen atom of the first molecule forming (— OH) group to give g-hydroxy aldehyde or ketone.
- This is a reversible reaction, establishing an equilibrium favouring aldol formation to a greater extent than ketol formation.
For aldehyde :
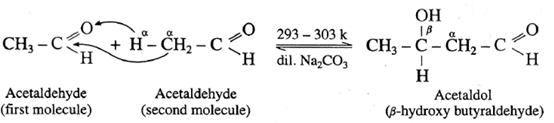
Acetaldol on heating undergoes subsequent elimination of water giving rise to α, β unsaturated aldehyde.
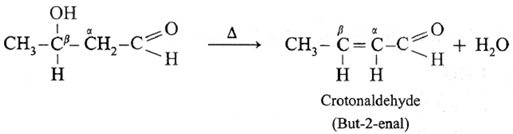
The overall reaction is called aldo] condensation. It is a nucleophilic addition-elimination reaction.
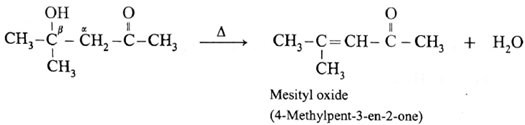
For ketone :
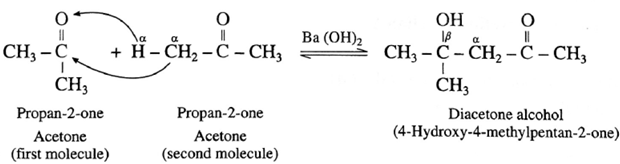
Diacetone alcohol on dehydration by heating forms α, β unsaturated ketone.
Cross aldol condensation :
- Cross aldol condensation refers to the aldol condensation that takes place in between two different aldehydes or ketones .
- If both aldehydes or ketones contain two α-hydrogen atoms each, then a mixture of four products, is formed.
- For example, a mixture of ethanal and propanal on reaction with dilute alkali followed by heating gives a mixture of four products.
Self aldol condensation :
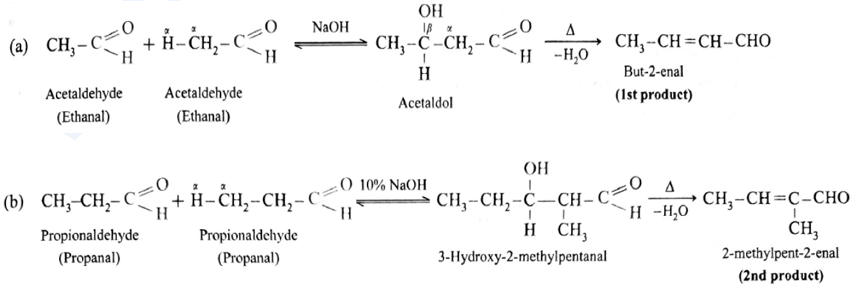
Cross aldol condensation :

- Ketones can also be used as one of the components in cross aldol condensation.
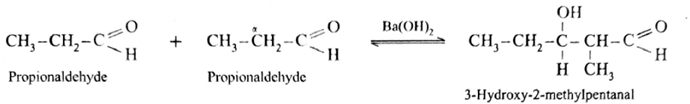
Aldol condensation reaction of propionaldehyde :
Since propionaldehyde has α -hydrogen atom it undergoes aldol condensation with alkali Ba(OH)2 forming 3-Hydroxy-2-methylpentanal.
3-Hydroxy-2-methylpentanal on heating undergoes dehydration and forms 2-Methylpent-2-enal.
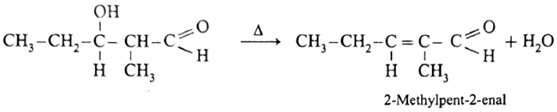
If a mixture of formaldehyde and acetaldehyde is subjected to aldol condensation :
- Since formaldehyde does not have a-hydrogen atom it will not undergo self aldol condensation.
- Since acetaldehyde has a-hydrogen atom, it will undergo self aldol condensation.
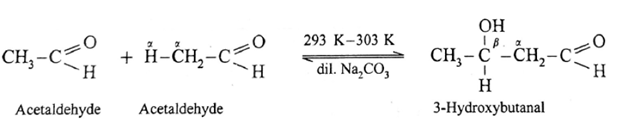
- Formaldehyde and acetaldehyde undergo cross aldol condensation.
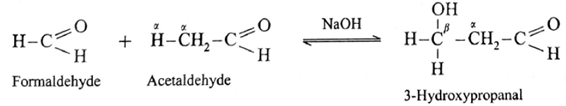
- Hence two aldol condensation products will be obtained.
(8) Cannizzaro reaction :
- Aldehydes which do not have a-hydrogen atom, on heating with concentrated alkali (50% aqueous or ethanolic solution of NaOH or KOH) undergo self oxidation and reduction reaction or redox reaction.
- This self redox reaction or disproportionation reaction is called Cannizzaro reaction.
- In this reaction one molecule of the aldehyde is oxidised to carboxylic acid while the second molecule of the aldehyde is reduced to alcohol (carboxylic acid formed, reacts with alkali, NaOH and forms a salt R—COONa).
- When formaldehyde (methanal) is heated with 50% NaOH solution, methanol (reduction product) and sodium formate (oxidation product) are formed.

Similarly, benzaldehyde gives Cannizzaro reaction.
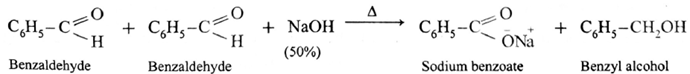
- Ketones and aldehydes like acetaldehyde, propionaldehyde, etc. having α-H atom do not give Cannizzaro reaction.
Cross Cannizzaro reaction :
- The reaction between two different aldehydes, not having a-hydrogen atoms is called cross Cannizzaro reaction.
- These two aldehydes undergo disproportionation in presence of concentrated alkali to give four products. However, if one of the aldehydes is formaldehyde, the reaction yields exclusively formate and alcohol to corresponding aldehyde.
- Formaldehyde and benzaldehyde since do not have a-hydrogen atom, will undergo Cannizzaro (redox) reactions.
Self Cannizzaro (redox) reaction :
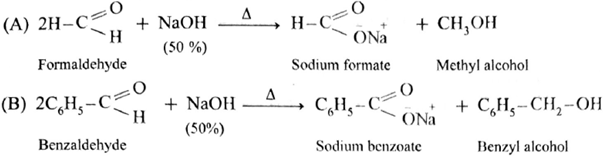
Cross Cannizzaro (redox) reaction :
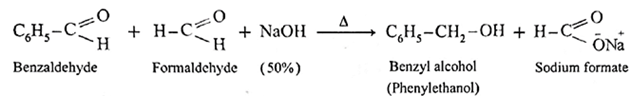
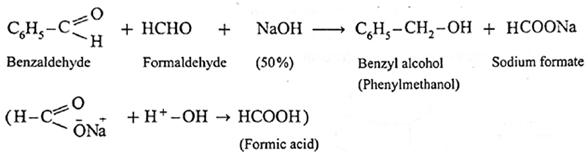
Example :
(i) Action of conc. potassium hydroxide on benzaldehyde :
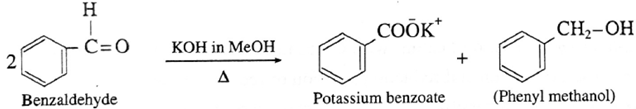
Question : Can isobutyraldehyde undergo Cannizzaro reaction ?
Since isobutyraldehyde contains a-carbon atom, it cannot undergo Cannizzaro reaction.
Differentiate between Cannizzaro reaction and Aldol reaction :
| Cannizzaro reaction | Aldol reaction |
| It is given by aldehydes not having alpha hydrogen atom | It is given by aldehydes and ketones possessing alpha hydrogen atom. |
| In this reaction an aldehyde is converted to the corresponding acid and an alcohol. | In this reaction aldehydes and ketones are converted into aldol and ketols, respectively. |
| It is a disproportionate ion reaction. | It is an addition reaction |
| It requires concentrated alkali as a catalyst. | It requires dilute alkali as a catalyst. |
Oxidation and reduction reactions of aldehydes and ketones ;
(1) Oxidation of aldehydes and ketones by dilute HNO3, KMnO4 and K2Cr2O7 :
(i) Aldehydes are oxidized to the corresponding carboxylic acids by oxidant such as dilute nitric acid, potassium permanganate and sodium or potassium dichromate in acidic medium.
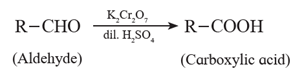
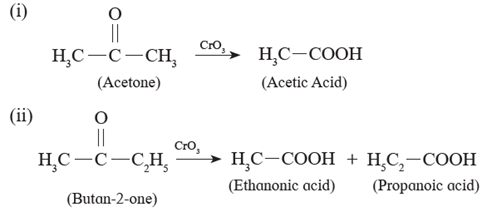
(ii) Ketones resist oxidation due to strong CO-C bond ,but they are oxidized by strong oxidizing agents such as CrO3, alkaline KMnO4 or hot concentrated HNO3 to a mixture of carboxylic acids having less number of carbon atoms than the starting ketone. Thus, Oxidation of ketones is accompanied by breaking C - C bond.
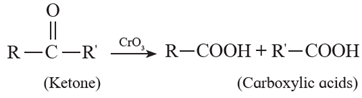 Examples :
Examples :
(2) Clemmensen and Wolf-Kishner reduction:
(a) Clemmensen reduction :
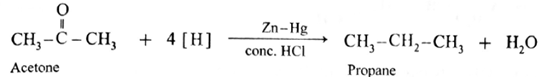
The carbonyl group (>C = 0) on reduction with zinc amalgam (Zn-Hg) in concentrated hydrochloric acid is converted into methylene group (—CH2—).
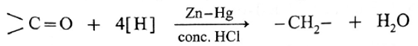
Aldehydes and ketones on reaction with Zn—Hg in concentrated HC forms corresponding alkanes. This reduction is called Clemmensen reduction.
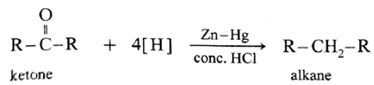
Acetaldehyde on reduction with Zn—Hg in concentrated HCI forms ethane.
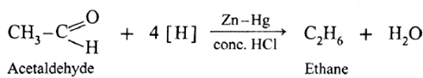
Acetone on reduction with Zn—Hg in concentrated HCI forms propane.
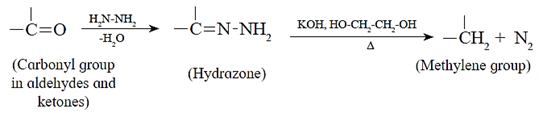
(b) Wolff—Kishner reduction :
Hydrazine (NH2.NH2) reduces carbonyl group (>C= O) of aldehydes or ketones to metylene group (>CH2). When aldehyde or ketone is heated with hydrazine in the presence of base such as potassium hydroxide and ethylene glycol, an alkane is obtained due to reduction of carbonyl compound.
Examples :
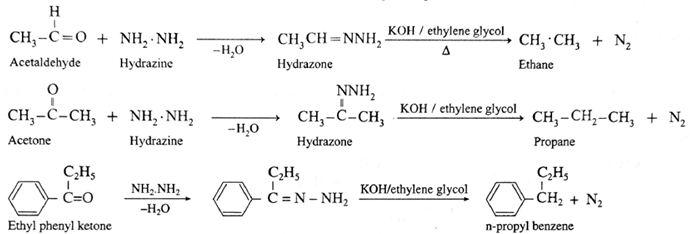
- Wolf-Kishner reduction is used to synthesize straight chain alkyl substituted benzenes which is not possible by Friedel-Crafts alkylation reaction.
(4) Electrophilic substitution reactions:
- Aromatic aldehydes and ketones undergo electrophilic substitution reactions such as nitration ,sulfonation and halogenation.
- The aldehydic ( -CHO) and ketonic (>C=O) groups are electron-withdrawing by inductive as well as resonance effects. They deactivate the benzene ring at ortho- and para- positions.
- This results in the formation of meta-
Example :
(i) Benzaldehyde on reaction with concentrated nitric acid in presence of conc. H2SO4 forms m-nitrobenzaldehyde
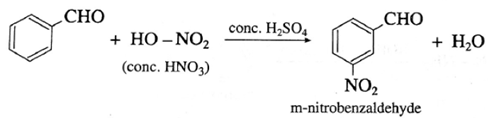
(2) Benzophenone on reaction with concentrated nitric acid in presence of conc. H2SO4 forms m-nitrobenzophenone
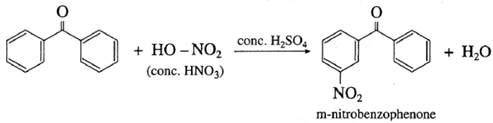
Chemical properties of carboxylic acids :
(1) Acidic character of carboxylic acids:
The carboxyl group (- COOH) imparts acidic character to carboxylic acids.
- A carboxyl group is made of -OH group bonded to a carbonyl group.
- In aqueous solution the H atom in OH of carboxyl group dissociates as proton and carboxylate ion is formed as the conjugate base,
![]()
- Carboxylate ion is resonance stabilized by two equivalent resonance structures.
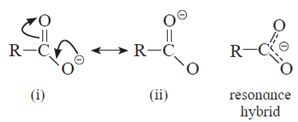
- Carboxylate ion has two resonance structures (i) and (ii) and both of them are equivalent to each other. This gives good resonance stabilization to carboxylate ion, which in turn gives acidic character to carboxylic acids.
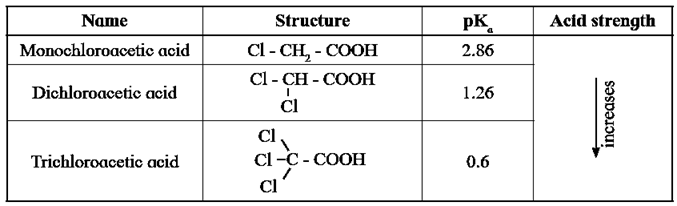
- Influence of electronic effects on acidity of carboxylic acids : All the carboxylic acids do not have the same pKa value. The structure of 'R' in R-COOH has influence on the acid strength of carboxylic acids. (Ref. below table)
- Halogens are electronegative atoms and exert electron withdrawing inductive effect (-I effect). The negatively charged carboxylate ion in the conjugate base of haloacetic acid gets stabilized by the -I effect of halogen. Which is responsible to diffuse the native charge.

- Higher the electronegativity of halogen greater is the stabilization of the conjugate base, stronger is the acid and smaller is the pKa value.
Table : pKa values of haloacetic acids

Acidity of aromatic carboxylic acids : Benzoic acid is the simplest aromatic acid.
From the pKa value of benzoic acid (4.2) is stronger than acetic acid (pKa 4.76).
- The sp2 hybrid carbon of aromatic ring exerts electron withdrawing inductive effect (-I effect) which stabilizes the conjugate base and increases the acid strength of aromatic acids.
- Electron–withdrawing groups like -Cl, -CN, and -NO2 increase the acidity of substituted benzoic acids while electron –donating group like –CH3, - OH , - OCH3 and -NH2 decrease the acidity of substituted benzoic acids .
Table : pKa values of chloroacetic acids :
Laboratory tests for carboxyl (—COOH) group :
The presence of -COOH group in carboxylic acids is identified by the following tests :
(i) Litmus test : (valid for water soluble substances) : Aqueous solution of Organic compound containing —COOH group turns blue litmus red which indicates the presence of acidic functional group.
(ii) Sedium bicarbonate test : When sodium bicarbonate is added to an organic compound containing —COOH group, a brisk effervescence of carbon dioxide gas is evolved. Water insoluble acid goes in solution and gives precipitate an acidification with conc. HCl. This indicates the presence of —COOH group.
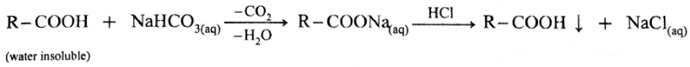
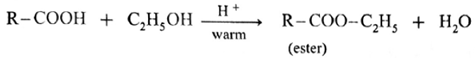
(iii) Ester test : One drop of concentrated sulfuric acid is added to a mixture of given organic compound containing -COOH group and one mL of ethanol, the reaction mixture is heated for 5 minutes in hot water bath. After this, hot solution is poured in a beaker containing water, fruity smell of ester confirms the presence of carboxylic acid.
Formation of acyl chloride :
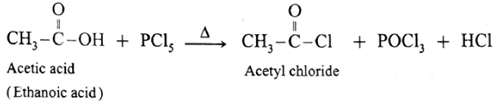
Reaction with PCl3, PCl5, SOCl2 : Carboxylic acids on heating with PCl3, PCl5, SOCl2 give the corresponding acyl chlorides. Thionyl chloride (SOCl2) is preferred because the byproducts formed are in gaseous state so they can easily escape from the reaction mixture. In this reaction –OH group of –COOH is replaced by –Cl .
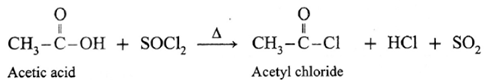
(a) Action on SOCl2 on carboxylic acid :
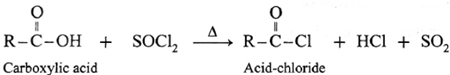
Example : Acetic acid reacts with thionyl chloride to give acetyl chloride.
(b) Action of PCl3 on carboxylic acid (ethanoic acid) :
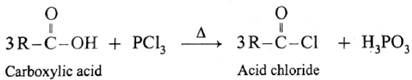
Example : Action of phosphorus trichloride on acetic acid gives acetyl chloride.
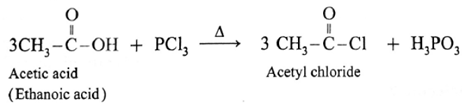
(c) Action of PCl5 on carboxylic acid :
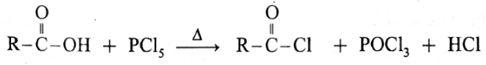
Reaction with ammonia : Formation of amide :
(i) Carboxylic acid or acid chloride with ammonia salts, which on further strong heating at high temperature decompose to give amides.
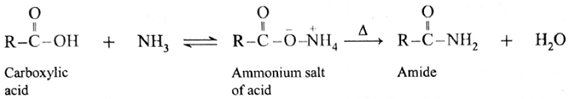
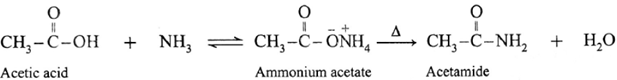
(ii) When acetic acid is treated with ammonia, ammonium acetate is obtained. Ammonium acetate on strong heating decomposes to form acetamide.
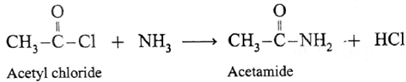
(iii) When acetyl chloride is treated with ammonia, acetamide is obtained
Formation of acid anhydride :
When carboxylic acid is heated with strong dehydrating agent like phosphorus pentoxide or concentrated sulphuric acid, an acid anhydride is obtained,
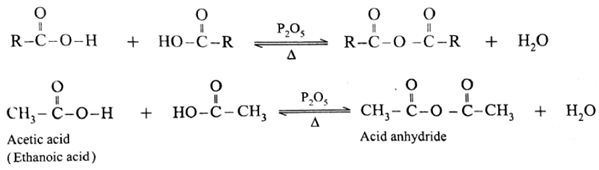
The reaction is reversible and anhydride is hydrolysed back to acid.
Alternatively, when sodium acetate is heated with acetyl chloride, acetic anhydride is obtained. This reaction is irreversible.
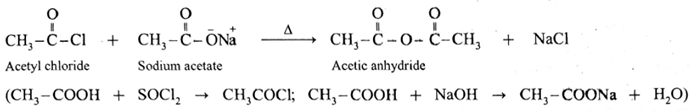
Decarboxylation of carboxylic acids :
Sodium salts of carboxylic acids on heating with soda lime give hydrocarbons which contain one carbon atom less than the carboxylic acid. For example When sodium acetate is heated with soda lime, methane is obtained.
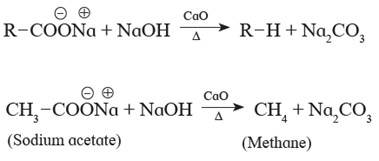
Reduction of carboxylic acids :
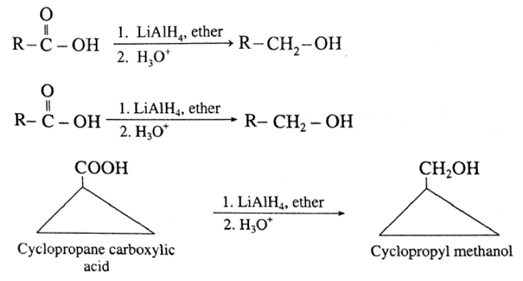
Carboxylic acids are reduced to primary alcohols by powerful reducing agent like lithium aluminium hydride. Carboxylic acid can also be reduced by diborane (diborane does not reduce –COOR , -NO2 , -X).
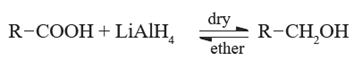
(Note : Sodium borohydride (NaBH4 )does not reduce-COOH group).
(i) When ethanoic acid is reduced in the presence of LiAIH4 in dry ether, forms ethanol.
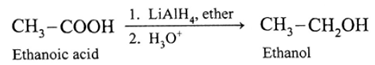
(ii) When cyclopropane carboxylic acid is reduced in the presence of lithium aluminium hydride in dry ether, forms cyclopropyl methanol.
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 11- Alcohols, Phenols and Ethers – Online Notes
Next Chapter : Chapter-13-Amines– Online Notes
We reply to valid query.