Thermodynamics
Maharashtra Board-Class-12th-Physics-Chapter-4
Notes-Part-2
Topics to be Learn : Part-2
|
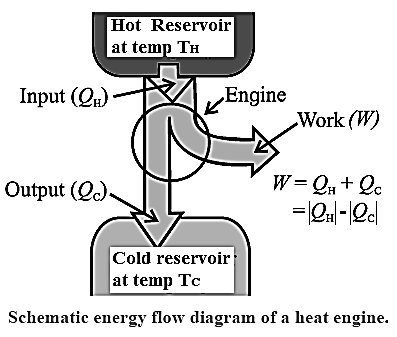
Heat engine
Heat engine : A heat engine is a device in which a system is taken through cyclic processes that result in converting part of heat supplied by a hot reservoir into work (mechanical energy) and releasing the remaining part to a cold reservoir. At the end of every cycle involving thermodynamic changes, the system is returned to the initial state. Example : Automobile engine is a heat engine.
A heat engine receives heat from a source called reservoir and converts some of it into work. All the heat absorbed by engine is not converted into work by a heat engine. Some heat is lost in the form of exhaust.
Elements of heat engine :
The following are the parts of a typical heat engine :
1) Working substance : It can be
- a mixture of fuel vapour and air in a gasoline (petrol) engine or diesel engine
- steam in a steam engine.
The working substance is called a system.
2) Hot and cold reservoirs : The hot reservoir is a source of heat that supplies heat to the working substance at constant temperature TH. The cold reservoir, also called the sink, takes up the heat released by the working substance at constant temperature TC < TH.
3) Cylinder and piston : The working substance is enclosed in a cylinder fitted with (ideally) a movable, massless, and frictionless piston. The walls of the cylinder are non-conducting, but the base is conducting. The piston is non-conducting. The piston is connected to a crank shaft so that the work done by the working substance (mechanical energy) can be transferred to the environment.
Types of heat engines :
- External combustion engine in which the working substance is heated externally as in a steam engine.
- Internal combustion engine in which the working substance is heated internally as in a petrol engine or diesel engine.
Working of a heat engine :
- The working substance absorbs heat (QH) from a hot reservoir at constant temperature. TH. It is an isothermal process QH is positive.
- Part of the heat absorbed by the working substance is converted into work (W), i.e. mechanical energy.
- In this case, there is a change in the volume of the substance.
- The remaining heat (|QC| = |QH| - W) is transferred to a cold reservoir at constant temperature TC < TH , QC is negative.
| The number of repetitions of the operating cycles of an automobile engine is indicated by its rpm or revolutions per minute. |
Thermal efficiency of a heat engine : In practical a heat engine do not convert all the heat absorbed, QH, in to work. There is always some heat lost, i.e., QC ≠ 0. The thermal efficiency η of the heat engine is defined as,
η = \(\frac{W}{Q_H}\), where W is the work done (output) by the working substance and QH is the amount of heat absorbed (input) by it.
Note : η has no unit and dimensions or its dimensions are [M°L°T°].
p-V diagram of a typical heat engine :
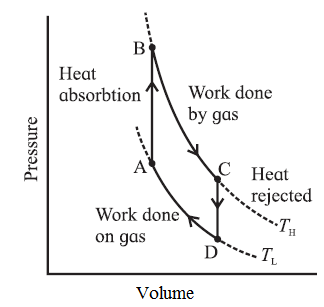
Here, TH is the temperature at which the work is done by the gas and Tc is the temperature at which the work is done on the gas. The area of the loop ABCDA is the work output.
Refrigerator and heat pump
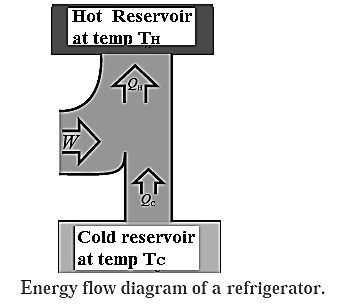
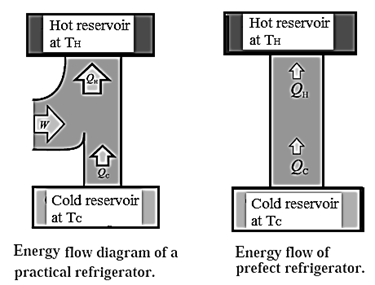
Refrigerator : A refrigerator is a device that uses work to transfer energy in the form of heat from a cold reservoir to a hot reservoir as it continuously repeats a thermodynamic cycle. Thus, it is a heat engine that runs in the backward direction.
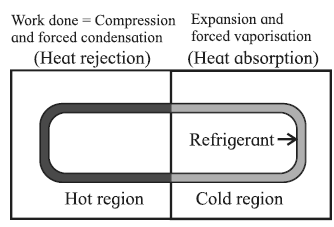
Working of a refrigerator :
A refrigerator performs a cycle in a direction opposite to that of a heat engine, the net result being absorption of some energy as heat from a reservoir at low temperature, a net amount of work done on the system and the rejection of a larger amount of energy as heat to a reservoir at a higher temperature.
The working substance undergoing the refrigeration cycle is called a refrigerant. The refrigerant (such as ammonia or Freon) is a saturated liquid at a high pressure and at as low a temperature as can be obtained with air or water cooling. The refrigeration cycle comprises the following processes :
- Throttling process : The saturated liquid refrigerant passes from the condenser through a narrow opening from a region of constant high pressure to a region of constant lower pressure almost adiabatically. It is a property of saturated liquids (not gases) that a throttling process produces‘ cooling and partial vaporization.
- Isothermal, isobaric vaporization— with the heat of vaporization being supplied by the materials or the region to be cooled : Heat QC is absorbed by the refrigerant at the low temperature Tc, thereby cooling the materials of the cold reservoir.
- Adiabatic compression of the refrigerant by an electrical compressor, thereby raising its temperature above TH.
- Isobaric cooling and condensation in the condenser at TH : In the condenser, the vapour is cooled until it condenses and is completely liquefied, i.e., heat QH is rejected to the surroundings which is the hot reservoir.
Refrigeration : Refrigeration is artificial cooling of a space or substance of a system and / or maintaining its temperature below the ambient temperature.
Steps through which a refrigerant goes in one complete cycle of refrigeration :
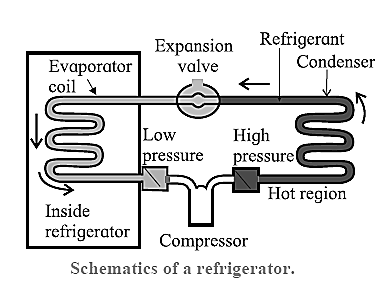
In one complete cycle of refrigeration, the refrigerant, a liquid such as fluorinated hydrocarbon, goes through the following steps.
- The refrigerant in the closed tube passes through the nozzle and expands, into a low—pressure area.
- This adiabatic expansion results in reduction in pressure and temperature of the fluid and the fluid tums into a gas.
- The cold gas is in thermal contact with the inner compartment of the fridge. Here it absorbs heat at constant pressure from the contents of the fridge.
- The gas passes to a compressor where it does work in adiabatic compression. This raises its temperature and converts it back into a liquid.
- The hot liquid passes through the coils on the outside of the refrigerator and releases heat to the air outside in an isobaric compression process.
The compressor, driven by an external source of energy, does work on the refrigerant during each cycle.
Performance of a Refrigerator :
In a refrigerator, QC is the heat absorbed by the working substance (refrigerant) at a lower temperature TC, W is the work done on the working substance, and QH is the heat rejected at a higher temperature TH. The absorption of heat is from the contents of the refrigerator and rejection of heat is to the atmosphere. Here, QC is positive and W and QH are negative. In one cycle, the total change in the internal energy of the working substance is zero.
∴ QH + QC = W, ∴ QH = W - QC
— QH = QC— W
Now, QH < 0, W < 0 and QC > O
|QH| = |QC| + |W|
The coefficient of performance (CoP), K, or quality factor, or Q value of a refrigerator is defined as
K = \(\frac{|Q_C|}{|W|}=\frac{|Q_C|}{|Q_C|-|Q_H|}\)
Note :K does not have unit and dimensions or its dimensions are [M°L°T°]
Remember :
|
Air conditioner : The working of an air conditioner is exactly similar to that of a refrigerator, but the volume of the chamber/ room cooled by an air conditioner is far greater than that in a refrigerator. The evaporator coils of an air conditioner are inside the room, and the condenser outside the room. A fan inside the air conditioner circulates cool air in the room.
The coefficient of performance, K of an air conditioner is defined as K = \(\frac{|Q_C|}{|W|}\) where QC is the heat absorbed and W is the work done. The time rate of heat removed is the heat current, H = \(\frac{|Q_C|}{t}\)
where t is the time in which heat |QC|, is removed.
K = \(\frac{|Q_C|}{|W|} = \frac{|Q_C|/t}{|W|/t}\)
where H = lQC|/t is the heat current and P(= |W|/ t) is the time rate of doing work, i.e., power.
| Capacity of an air conditioner is expressed in tonne. Do you know why?
Before refrigerator and AC was invented, cooling was done by using blocks of ice. When cooling machines were invented, their capacity was expressed in terms of the equivalent amount of ice melted in a day (24 hours). The same term is used even today. |
Heat Pump: Heat pump is a device which works similar to a refrigerator.
- It is used to heat a building or a similar larger structure by cooling the air outside it.
- A heat pump works like a refrigerator operating inside out.
- In this case, the evaporator coils are outside and absorb heat from the cold air from outside.
- The condenser coils are inside the building. They release the absorbed heat to the air inside the thus, warming the building.
| Remember :
Heat flow from a hot object to a cold object is spontaneous whereas, work is always required for the transfer of heat from a colder object to a hotter object. |
Second Law of Thermodynamics
Limitations of the First Law of Thermodynamics:
- The first law of thermodynamics is essentially the principle of conservation of energy as there is a close relation between work and energy. We find that there is a net transfer of energy (heat) from a body at higher temperature to a body at lower temperature. The net transfer of energy from a body at lower temperature to a body at higher temperature is not observed though consistent with the first law of thermodynamics.
- If two containers, one containing nitrogen and the other containing oxygen, are connected to allow diffusion, they mix readily. They never get separated into the respective containers though there is no violation of the first law of thermodynamics.
- It is not possible to design a heat engine with 100% efficiency, though there is no restriction imposed by the first law of thermodynamics.
- At room temperature, ice absorbs heat from the surrounding air and melts. The process in the opposite direction is not observed, though consistent with energy conservation. These examples suggest that there is some other law operative in nature that determines the direction of a process.
Second law of thermodynamics :
- It is impossible to extract an amount of heat QH from a hot reservoir and use it all to do work W.
- Some amount of heat QC must be exhausted (given out) to a cold reservoir. This prohibits the possibility of a perfect heat engine.
- This statement is called the first form or the engine law or the engine statement or the Kelvin—Planck statement of the second law of thermodynamics. K
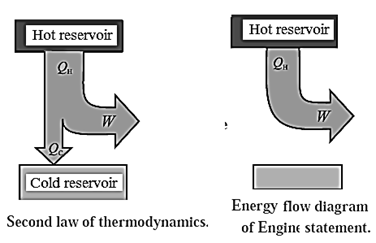
- It is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this. This prohibits the possibility of a perfect refrigerator.
- This statement is called the second form or the Clausius statement of the second law of thermodynamics.
The Second law of thermodynamics is a general principle which puts constraints upon the direction of heat transfer and the efficiencies that a heat engine can achieve.
| Remember :
When we say that energy will not flow spontaneously from a cold object to a hot object, we are referring to the net transfer of energy. Energy can transfer from a cold object to a hot object either by transfer of energetic particles or electromagnetic radiation. However, in any spontaneous process, the net transfer of energy will be from the hot object to the cold object. Work is required to transfer net energy from a cold object to a hot object. |
Carnot cycle and Carnot engine
Significance of Reversibility in Thermodynamics:
- A reversible process is a bidirectional process, i.e. its path in P — V plane is the same in either direction. In contrast, an irreversible process is a unidirectional process, i.e., it can take place only in one direction.
- A reversible process consists of a very large number of infinitesimally small steps so that the system is all the time in thermodynamic equilibrium with its environment. In contrast an irreversible process may occur so rapidly that it is never in thermodynamic equilibrium with its environment.
Maximum Efficiency of a Heat Engine and Carnot’s Cycle:
Basically, two processes occur in a Carnot engine
Exchange of heat with the reservoirs : In isothermal expansion AB, the working substance takes in heat QH from a lot reservoir (source) at constant temperature TH. In isothermal compression CD, the working substance gives out heat QC to a cold reservoir (sink) at constant temperature TC.
Doing work : In adiabatic expansion BC, the working substance does work on the environment and in adiabatic compression DA, work is done on the working substance by the environment.
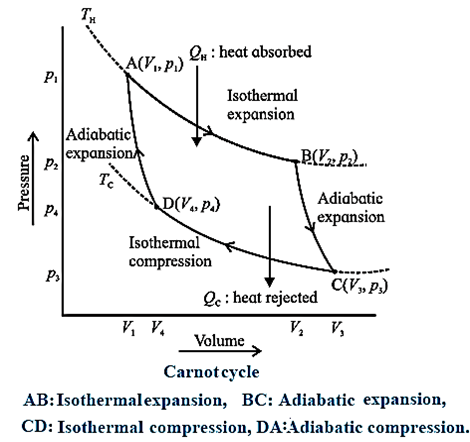
All processes are reversible. It can be shown that
\(\frac{|Q_C|}{|Q_H|}=\frac{T_C}{T_H}\)= Hence, the efficiency of a Carnot engine,
η = \(\frac{W}{Q_H} = \frac{Q_H-|Q_C|}{Q_H}\)
= \(1-|\frac{Q_C}{Q_H}|=1-\frac{T_C}{T_H}\)
The temperature of the cold reservoir sets the limit on the efficiency of a heat engine.
η = \(1-\frac{T_C}{T_H}\) This formula shows that for maximum efficiency, TC should be as low as possible and TH should be as high as possible.
For a Carnot engine, efficiency T
η = \(1-\frac{T_C}{T_H}\), η →1 as TC → 0
Q. Suggest a practical way to increase the efficiency of a heat engine.
The efficiency of a heat engine can be increased by choosing the hot reservoir at very high temperature and cold reservoir at very low temperature.
Remember for a Carnot Engine:
Important :
|
Carnot refrigerator :A Carnot refrigerator is a Carnot engine operated in the reverse direction. Here, heat QC is absorbed from a cold reservoir at temperature TC, work W is provided externally, and heat QH is given out to a hot reservoir at temperature TH.
The coefficient of performance of a Carnot refrigerator :
K = \(\frac{|Q_C|}{|W|}=\frac{|Q_C|}{|Q_C|-{|Q_H|}}\)
= \(\frac{T_C}{T_H-T_C}\) as \(\frac{|Q_C|}{|Q_H|}=\frac{T_C}{T_H}\)
- K is large if TH—TC is small. It means a large quantity of heat can be removed from the body at lower temperature to the body at higher temperature by doing small amount of work.
- K is small if TH—TC is large. It means a small quantity of heat can be removed from the body at lower temperature to the body at higher temperature even with large amount of work
Sterling Cycle
Sterling cycle and the various processes taking place in a Sterling engine :
The working substance can be air or helium or hydrogen or nitrogen. All processes are reversible.
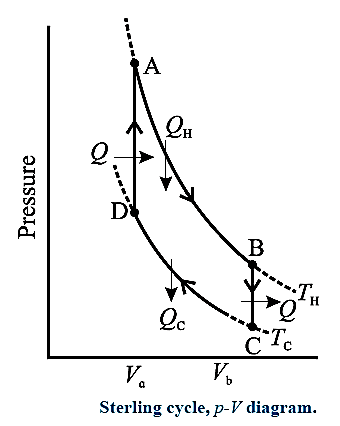
- AB is isothermal expansion, at temperature TH, in which heat QH is absorbed from the source and useful work is done by the working substance. [In this case, the change in the internal energy is zero, as the temperature of the gas remains constant. Hence, the work done, W = heat absorbed, QH.]
- BC is isochoric process in which some heat is released by the gas (working substance) to the refrigerator and the gas cools to temperature TC < TH. [In this case, the change in the internal energy, ΔU = nCv (TC — TH), where n = number of moles of the gas used in the Sterling engine and CV = molar specific heat of the gas. As W = 0 at constant volume, heat released = ΔU.]
- CD is isothermal compression, at temperature Tc , in which heat QC is rejected to the coolant (sink).
- DA is isochoric process in which heat is taken in by the gas and its temperature rises to TH.
Note : Sterling engine operated in reverse direction is used in the field of cryogenics to obtain extremely low temperatures to liquefy air or other gases.
First law of thermodynamics : ΔU = Q— W.
Equation of state of an ideal gas : PV = nRT
Work done by a gas, W = \(\int_{V_i}^{V_f}Pdv\)
Isobaric process (P = constant), W = P (Vf— Vi) = nR (Tf— Ti), Isochoric process, (V = constant), W = 0
Isothermal process, (T = constant), W = nRT\(ln\frac{V_f}{V_i}\) = nRT\(ln\frac{P_i}{P_f}\)
Adiabatic process, PVγ = constant, W = \(\frac{nR(T_i-T_f)}{\gamma -1}= \frac{P_iV_i-P_fV_f}{\gamma -1}\)
Change in internal energy, ΔU = nCv(Tf—Ti)
Heat supplied, Q = nCP (Tf—Ti)
Carnot engine efficiency η = \(\frac{W}{Q_H}=\frac{Q_H-|Q_C|}{Q_H}=\frac{T_H-T_C}{T_H}\)
Carnot refrigerator : Coefficient of performance, K = \(\frac{Q_C}{W}=\frac{Q_C}{|Q_H|-Q_C}=\frac{T_C}{T_H-T_C}\)
Air conditioner :
- Heat current, H = \(\frac{Q_C}{t}\)
- Power, P = W/t
- Coefficient of performance, K = \(\frac{Q_C}{W}=\frac{Ht}{Pt}=\frac{H}{P}\)
Download from store : Chapter-4-Thermodynamics-Text Book Get PDF from store : Chapter-4-Thermodynamics-Notes Get PDF from store : Chapter-4-Thermodynamics-Solution
Main Page : – Maharashtra Board Class 12th-Physics – All chapters notes, solutions, videos, test, pdf. Previous Chapter : Chapter-3-Kinetic Theory of Gases and Radiations –Online Notes Next Chapter : Chapter-5-Oscillations – Online Notes
We reply to valid query.