Coordination Compounds
Maharashtra Board-Class-12-Chemistry-Chapter-9
Notes-Part-2
Topics to be Learn : Part-2
|
Stability of the coordination compounds:
- The stability of coordination compounds can be explained by knowing their stability constants.
- The stability is governed by metal-ligand interactions.
- In this the metal serves as electron-pair acceptor while the ligand as Lewis base (since it is electron donor).
- The metal-ligand interaction can be realized as the Lewis acid-Lewis base interaction. Stronger the interaction greater is stability of the complex.
Consider the equilibrium for the metal ligand interaction :
Ma+ + nLx— ⇌ [MLn](a+)+(nx—)
where a, x, [a+ + nx- ] denote the charge on the metal, ligand and the complex, respectively.
Now, the equilibrium constant K is given by
K = \(\frac{[ML_n]^{(a+)+(nx-)}}{[M^{a+}][L^{x-}]_n}\)
Stability of the complex can be explained in terms of K. Higher the value of K larger is the thermodynamic stability of the complex.
The equilibria for the complex formation with the corresponding K values are given below.
Ag+ + 2CN ⇌ [Ag(CN)2] — K = 5.5 ×1018
Cu2+ + 4CN— ⇌ [Cu(CN)4]2— K = 2.0 ×1027
Co3+ + 6NH3 ⇌ [Co(NH3)6]3+ K = 5.0 ×1033
From the above data, [Co(NH3)6]3+ is more stable than [Ag(CN)2] — and [Cu(CN)4]2—.
Factors which govern stability of the complex : Stability of a complex is governed by (a) charge to size ratio of the metal ion and (b) nature of the ligand.
- Charge to size ratio of the metal ion : Higher the ratio greater is the stability. For the divalent metal ion complexes their stability shows the trend : Cu2+ > Ni2+ > Co2+ > F2+ > Mn2+ > Cd2+ . The above stability order is called Irving-William order. In the above list both Cu and Cd have the charge + 2, however, the ionic radius of Cu2+ is 69 pm and that of Cd2+ is 97 pm. The charge to size ratio of Cu2+ is greater than that of Cd2+. Therefore the Cu2+ forms stable complexes than Cd2+.
- Nature of the ligand : A second factor that governs stability of the complexes is related to how easily the ligand can donate its lone pair of electrons to the central metal ion that is, the basicity of the ligand. The ligands those are stronger bases tend to form more stable complexes.
Theories of bonding in complexes :
The metal-ligand bonding in coordination compounds has been described by Valence bond theory (VBT) and Crystal field theory (CFT).
Valence bond theory (VBT) :
- Vacant d-orbitals of metal ion form coordination bonds with ligands.
- s, p orbitals along with vacant d—orbitals of metal ion take part in hybridisation.
- The number of vacant hybrid orbitals formed is equal to number of hybridising orbitals which is equal to the number of ligand donor atoms or coordination number of the metal.
- The metal-ligand coordination bonds are formed by the overlap between the vacant hybrid orbitals of metal and the filled orbitals of the ligands.
- The hybrid orbitals used by the metal ion point in the direction of the ligands.
- When inner (n — 1)d orbitals of metal ion are used in the hybridisation then the complex is called (a) inner orbital complex while when outer nd orbitals are used, complexes are called (b) outer orbital complexes.
Steps to understand the metal-ligand bonding :
- Find oxidation state of central metal ion
- Write valence shell electronic configuration of metal ion.
- See whether the complex is low spin or high spin. (applicable only for octahedral complexes with d4 to d8 electronic configurations).
- From the number of ligands find the number of metal ion orbitals required for bonding.
- Identify the orbitals of metal ion available for hybridisation and the type of hybridisation involved.
- Write the electronic configuration after hybridisation.
- vii Show filling of orbitals after complex formation.
- viii.Determine the number of unpaired electrons and predict magnetic behavior of the complex.
Salient features of valence bond theory (VBT) :
- According to this theory, a central metal atom or ion present in a complex provides a definite number of vacant orbitals (s, p, d and f ) to accommodate the electrons from the ligands for the formation coordinate bonds with the metal ion / atom.
- The number of vacant orbitals provided by the central metal atom or ion is the same as the coordination number of the metal. For example : Cu2+ provides 4 vacant orbitals in the complex. [Cu(NH3)4] 2+.
- The vacant orbitals of metal atom or ion undergo hybridisation forming the same number of hybridised orbitals, since the bonding with the hybrid orbitals is stronger.
- Each ligand has one or more orbitals containing one or more lone pairs of the electrons.
- The shape or geometry of the complex depends upon the type of hybridisation of the metal ion / atom.
- When inner orbitals namely (n — 1) d orbitals in transition metal atom or ion hybridise, the complex is called inner complex and when outer orbitals i.e., nd orbitals hybridise then the complex is called outer complex.
- When the central metal atom or ion in the complex contains one or more unpaired electrons the complex is paramagnetic while if all the electrons are paired, the complex is diamagnetic.
Strong ligands and weak ligands :
When the ligands approach the metal atom or ion for the formation of a complex, they influence the valence electrons of metal atom or ion. Accordingly the ligands are classified as (A) strong ligands and (B) weak ligands.
Strong ligands :
They cause the pairing of unpaired electrons present in the metal atom or ion.
Strong field ligands are those in which donor atoms are C, N or P. Thus CN—, NC—, CO, HN3, EDTA, en (ethylenediammine) are considered to be strong ligands.
Spin pairing process :
- The process of pairing of unpaired electrons in metal atom or ion due to the presence of ligands in the complex is called spin pairing process.
- This spin pairing process decreases the number of unpaired electrons and hence decreases the paramagnetic character of the complex.
- The strong ligands also promote the outer ns electrons to the vacant inner (n — 1)d orbitals.
Weak ligands :
- The weak ligands have no effect on the electrons in the valence shell of a metal atom or ion.
- Weak field ligands are those in which donor atoms are halogens, oxygen or sulphur. For example,
- F—, Cl—, Br—, l—, SCN—, C2O42—. In case of these ligands the Δ0 parameter is smaller compared to the energy required for the pairing of electrons, which is called as electron pairing energy.
Octahedral, complexes :
Structure of octahedral complex, [CO(NH3)6]3+ on the basis of valence bond theory : (low spin)
(i) Hexaamminecobalt(III) ion, [CO(NH3)6]3+ is a cationic complex, the oxidation state of cobalt is +3 and the coordination number is 6.
(ii) Electronic configuration : 27Co [Ar]18 3d7 4s2
Electronic configuration : Co3+ [Ar]18 3d6 4s0 4p0
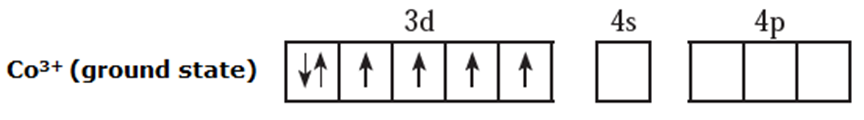
(iii) Since NH3 is a strong ligand, due to spin pairing effect, all the four unpaired electrons in 3d orbital are paired giving two vacant 3d orbitals.
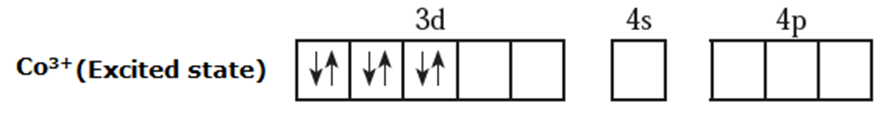
(iv) Since the coordination number is 6, Co3+ ion gets six vacant orbitals by hybridisation of two 3d vacant orbitals, one 4s and three 4p orbitals forming six d2sp3 hybrid orbitals giving octahedral geometry It is an inner complex.
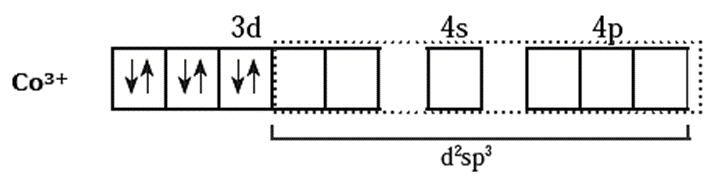
(v) 6 lone pairs of electrons from 6NH3 ligands are accommodated in the six vacant d2sp3 hybrid orbitals. Thus six hybrid orbitals of Co3+ overlap with filled orbitals of NH3 forming 6 coordinate bonds giving octahedral geometry to the complex.
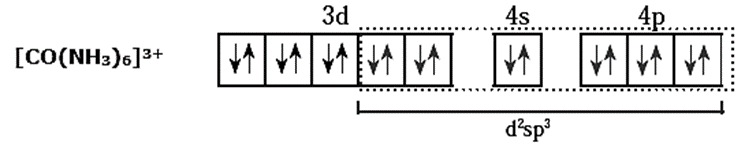
(vi) As all electrons are paired the complex is diamagnetic.
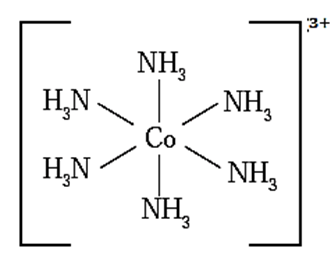
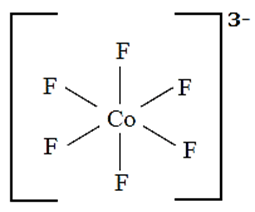
Geometry of [CoF6]3— (high spin) on the basis of valence bond theory :
(i) Oxidation state of central metal Co is 3+
(ii) Valence shell electronic configuration of Co3+ is
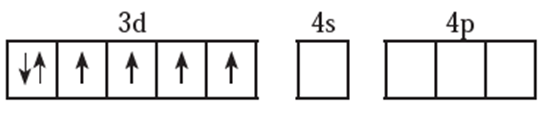
(iii) Six fluoride F ligands, thus the number of vacant metal ion orbitals required for bonding with ligands would be six.
(iv) Complex is high spin, that means pairing of electrons will not take place prior to
hybridisation. Electronic configuration would remain the same as in the free state shown above.
(v) Six orbitals available for the hybridisation. Those are one 4s, three 4p, two of 4d orbitals
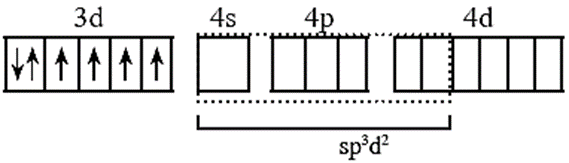
Six metal orbitals after bonding with six F ligands led to the sp3d2 hybridization. The d orbitals participating in hybridisation for this complex are nd.
(vi) Six vacant sp3d2 hybrid orbital of Co3+ overlap with six orbitals of fluoride forming Co - F coordinate bonds.
(vii) Configuration after complex formation.
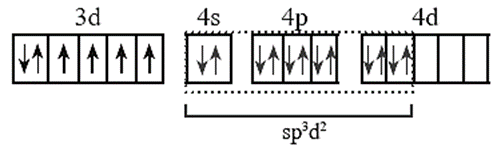
(viii) The complex is octahedral and has four unpaired electrons and hence, is paramagnetic.
Tetrahedral complex :
Structure of tetrachloronickelate(II) [Ni(Cl)4]2—on the basis of valence bond theory :
(i) Oxidation state of nickel is +2
(ii) Valence shell electronic configuration of Ni2+
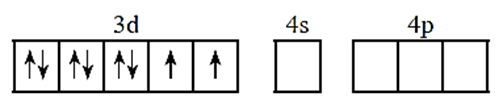
(iii) number of Cl— ligands is 4. Therefore number of vacant metal ion orbitals required for bonding with ligands must be four.
(iv) Four orbitals on metal available for hybridisation are one 4s, three 4p. The complex is tetrahedral.
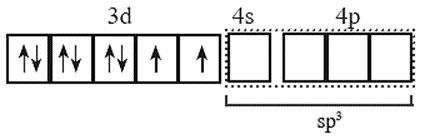
The four metal ion orbitals for bonding with Cl— ligands are derived from the sp3 hybridization.
(vi) Four vacant sp3 hybrid orbital of Ni2+ overlap with four orbitals of Cl— ions.
(vii) Configuration after complex formation would be.
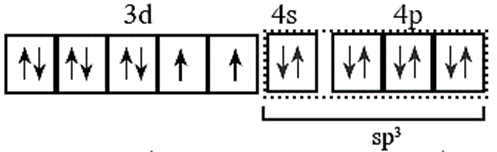
(viii)The complex has four unpaired electrons and hence, paramagnetic.
Magnetic moment μ is, μ = \(\sqrt{n(n+2)}=\sqrt{2(2+2)}\) = 2.83 B.M
Square planar complex :
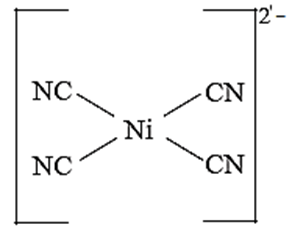
Structure of [Ni(CN)4]2—on the basis of valence bond theory :
(i) Oxidation state of nickel is +2
(ii) Valence shell electronic configuration of Ni2+
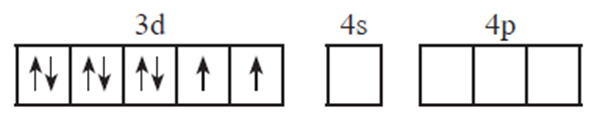
(iii) Number of CN ligands is 4, so number of vacant metal ion orbitals required for bonding with ligands would be four.
(iv) Complex is square planar so Ni2+ ion uses dsp2 hybrid orbitals.
(v) 3d electrons are paired prior to the hybridisation and electronic configuration of Ni2+ becomes :
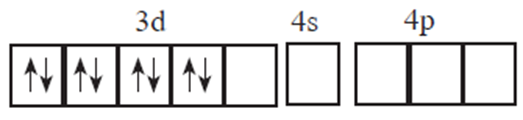
(vi) Orbitals available for hybridisation are one 3d, one 4s and two 4p which give dsp2 hybridization.
(vii) Four vacant dsp2 hybrid orbitals of Ni2+ overlap with four orbitals of CN ions to form Ni - CN coordinate bonds.
(vii) Configuration after the complex formation becomes.
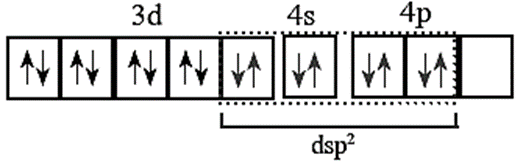
(viii) The complex has no unpaired electrons and hence, dimagnetic.
Remember :
| Coordination
number |
Geometry
of complex |
Hybridization |
| 2 | Linear | sp |
| 4 | Tetrahedral | sp3 |
| 4 | Square planar | dsp2 |
| 6 | Octahedral | d2sp3/ sp3d2 |
Limitations of valence bond theory :
In case of the coordination compounds, the valence bond theory has the following limitations :
- It cannot explain the complex chemicals' spectral characteristics (colours).
- Even though magnetic moments can be estimated from the number of unpaired electrons, this does not explain magnetic moments caused by electron orbital motion.
- It cannot explain why metal ions with the same oxidation state form inner complexes with distinct ligands and outer complexes with different ligands.
- The geometry of the complex cannot explain magnetic characteristics in any complex.
- Quantitative interpretations of thermodynamic and kinetic stabilities of the coordination compounds cannot be accounted.
- The complexes with weak field ligands and strong field ligands cannot be distinguished.
- It cannot predict the tetrahedral and square planar geometry of complexes with coordination number 4.
- The order of reactivity of inner complexes of d3, d4, d5 and d6 metal ions cannot be explained.
- It cannot explain the rates and mechanisms of reactions of the coordination compounds.
Crystal Field Theory (CFT) :
Assumptions of Crystal Field Theory (CFT) :
Bethe and van Vleck developed Crystal Field Theory (CFT) to explain various properties of coordination compounds. The salient features of CFT are as follows :
In a complex, the central metal atom or ion is surrounded by various ligands which are either negatively charged ions (F—, Cl—, CN—, etc.) or neutral molecules (H2O, NH3, en, etc.) and the most electronegative atom in them points towards central metal ion. .
The ligands are treated as point charges involving purely electrostatic attraction between them and metal ion.
- The central metal ion has five, (n — 1)d degenerate orbitals namely dxy, dyz, dzx, d(x2 – y2) and dz2.
- When the ligands approach the metal ion, due to repulsive forces, the degeneracy of d-orbitals is destroyed and they split into two groups of different energy, t2g and eg orbitals. This effect is called crystal field splitting which depends upon the geometry of the complex.
- The d-orbitals lying in the direction of ligands are affected to a greater extent while those lying in between the ligands are affected to a less extent.
- Due to repulsion, the orbitals along the axes of ligands acquire higher energy while those lying in between the ligands acquire less energy.
- Hence repulsion by ligands give two sets of split up orbitals of metal ion with different energies.
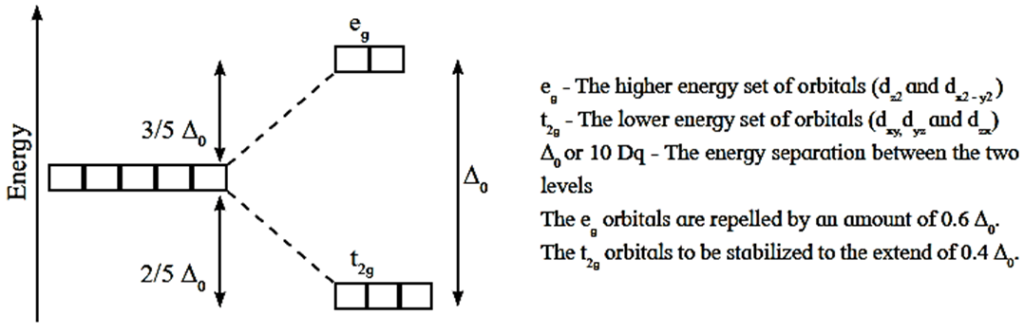
Crystal field Splitting in an octahedral complex
- The energy difference between two sets of d-orbitals after splitting by ligands is called crystal field splitting energy (CFSE) and represented by Δ0 or by arbitrary term 10Dq. The value of Δ or 10Dq depends upon the geometry of the complex.
The electrons of metal ion occupy the split d-orbitals according to Hund’s rule, aufbau principle and those orbitalswith minimum repulsion and the farthest away from the ligands.
CFT does not account for overlapping of orbitals of central metal ion and ligands, hence does not consider covalent nature of the complex.
From the crystal field stability energy, the stability of the complexes can be known.
Crystal field splitting :
The splitting of five degenerate d-orbitals of the transition metal ion into different sets of orbitals (t2g and eg)having different energies in the presence of ligands in the complex is called crystal field splitting.
Crystal field stabilisation energy ((CFSE) : It is the change in energy achieved by preferential filling up of the orbitals by electrons in the complex of metal atom or ion.
- CFSE is expressed as a negative quantity i.e., CFSE ≤ 0. Higher the negative value more is the stability of the complex.
Factors affecting Crystal Field Splitting parameter (Δ0)
Crystal Field Splitting parameter (Δ0) depends on, (a) Strength of the ligands and (b) Oxidation state of the metal.
(i) Strength of the ligands : The magnitude of crystal field splitting depends on strength of the ligands. The strong ligands those appear in spectrochemical series approach closer to the central metal which results in a large crystal field splitting.
- Strong field ligands like CN—, en, etc. approach closer to the central metal ion, it results in a large crystal field splitting and hence Δ0 has higher values.
(ii) Oxidation state of the metal : A metal with the higher positive charge is able to draw ligands closer to it than that with the lower one. Thus the metal in higher oxidation state results in larger separation of t2g and eg set of orbitals. The trivalent metal ions cause larger crystal field splitting than corresponidng divalent ones.
- For example [Fe(NH3)6]3+ has higher Δ0 than [Fe(NH3)6]2+
Colour of the octahedral complexes:
- A large number of coordination compounds show Wide range of colours due to a’-d transition of electron and this can be explained by crystal field theory (CFT).
- The complex absorbs the light in one visible region (400 nm to 700 nm) and transmits the light in different visible region giving complementary colour.
- Consider an octahedral purple coloured complex of [Ti(H2O)6]3+ which absorbs green light and transmits purple colour. Similarly [Cu(H2O)6] 2+ absorbs the light in the red region of radiation spectrum and transmits in the blue region, hence the complex appears blue.
- The absorption of light arises due to d-d transition of electron from lower energy level (t2g) to higher energy level (eg) in octahedral -complex.
- The energy required for transition depends upon crystal field splitting energy Δ0. If Δ0 = ΔE, then the energy of an absorbed photon (hv) is
- ΔE = hv = hc/λ
- where λ, v and c are wavelength, frequency and velocity of the absorbed light.
- Higher the magnitude of A0 or AE, higher is the frequency or lower is the wavelength of the absorbed radiation.
- Since A0 depends upon nature of metal atom or ion, its oxidation state, nature of ligands and the geometry of the complex, different coordination compounds have different colours.
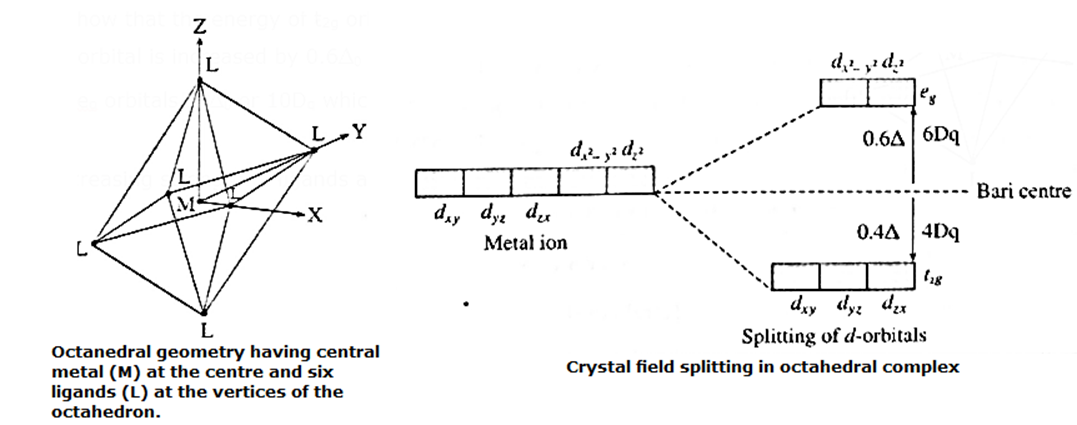
Octahedral geometry of complexes using crystal field theory :
- In an octahedral complex [MX6] ±, the metal atom or ion is placed at the centre of regular octahedron while six ligands occupy the positions at six vertices of the octahedron.
- Among five degenerate d-orbitals, two orbitals namely d(x2 – y2) and dz2; are axial and have maximum electron density along the axes, while remaining three d-orbitals namely dxy, dyz and dzx are planar and have maximum electron density in the planes and in-between the axes.
- Hence, when the ligands approach a metal ion, the orbitals d(x2 – y2) and dz2 experience greater repulsion and the orbitals dxy, dyz and dzx experience less repulsion.
- Therefore the energy of d(x2 – y2) and dz2 increases while the energy of dxy, dyz and dzx decreases and five d-orbital lose degeneracy and split into two point groups.
- The orbitals dxy, dyz and dzx form t2g group of lower energy while d(x2 – y2) and dz2 form eg group of higher energy.
- Thus t2g has three degenerate orbitals while eg has two degenerate orbitals.
- Experimental calculations show that the energy of t2g orbitals is lowered by 0.4 Δ0 or 4Dq and energy of eg orbital is increased by 0.6Δ0 or 6Dq. Thus energy difference between t2g and eg orbitals is Δ0 or 10Dq which is crystal field splitting energy.
- CFSE increases with the increasing strength of ligands and oxidation state of central metal ion
Tetrahedral geometry of complexes using crystal field theory :
The tetrahedral structure having the metal atom M at the centre and four ligands occupying the corners is displayed along with in Fig.
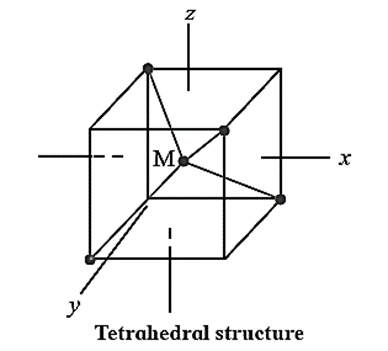
- The dxy, dyz and dzx orbitals with their lobes lying in between the axes point toward the ligands. On the other hand, d(x2 – y2) and dz2 orbitals lie in between metal-ligand bond axes.
- The dxy, dyz and dzx orbitals experience more repulsion from the ligands compared to that by d(x2 – y2) and dz2 orbitals.
- Due to larger such repulsions the dxy, dyz and dzx orbitals are of higher energy while the d(x2 – y2) and dz2 orbitals are of relatively lower energy.
- Each electron entering in one of dxy, dyz and dzx orbitals raises the energy by 4Dq whereas that accupying d(x2 – y2) and dz2 orbitals lowers it by 6Dq compared to the energy of hypothetical degenerate d orbitals in the ligand field.
- A splitting of d orbitals in tetrahedral crystal fields (assumed to be 10Dq) thus is much less (typically 4/9) compared to that for the octahedral environment.
- The crystal field splitting of d orbitals in a tetrahedral ligand field is compared with the octahedral one in below Fig.
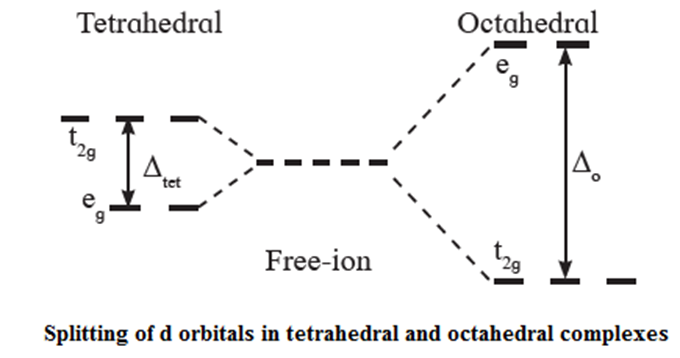
- Thus the pairing of electrons is not favoured in tetrahedral structure. For example, in d4 configuration an electron would occupy one of the t2g orbitals. The low spin tetrahedral complexes thus are not found.
- Typically metal complexes possessing the cetral metal ion with d8 electronic configuration, for example, Ni(CO)4, favours the tetrahedral structure.
High spin and low spin Complexes :
(1) High spin complex (HS) :
- The complex which has greater number of unpaired electrons and hence higher value of resultant spin and magnetic moment is called high spin (or spin free) or HS complex.
- It is formed with weak field ligands and the complexes have lower values for crystal field splitting energy (CFSE), Δ0.
- The paramagnetism of HS complex is larger.
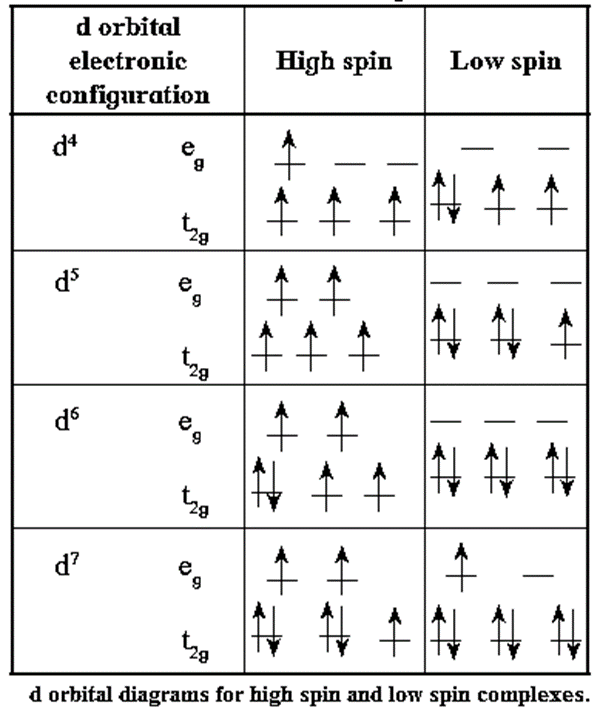
(2) Low spin complex (LS) :
- The complex which has the least number of unpaired electrons or all electrons paired and hence the lowest (or no) resultant spin or magnetic moment is called low spin (or spin paired) or LS complex.
- It is formed with strong field ligands and the complexes have higher values of crystal field splitting energy (Δ0).
- Low spin complex is diamagnetic or has low paramagnetism.
Applications of coordination compounds
In biology :
- Several biologically important natural compounds are metal complexes. They play important role in a number of processes occuring in plants and animals.
- For example, chlorophyll present in plants is a complex of Mg. Haemoglobin present in blood is a complex of iron.
In medicines :
- Pt complex cisplatin is used in the treatment of cancer.
- EDTA is used for treatment of lead poisoning.
To estimate hardness of water :
- Hardness of water is due to the presence of Ca2+ and Mg2+ ions.
- The ligand EDTA forms stable complexes with Ca2+ and Mg2+.
- It can, therefore, be used to estimate hardness.
Electroplating :
- This involves deposition of a metal on the other metal. For smooth plating, it is necessary to supply continuously the metal ions in small amounts.
- For this purpose, a solution of a coordination compound is used which dissociates to a very less extent.
- For example, for uniform and thin plating of silver and gold, the complexes K[Ag(CN)2] and K [Au(CN)2] are used.
PDF : Chapter-9-Coordination Compounds-Text Book
PDF : Chapter-9-Coordination Compounds- Notes
PDF : Chapter-9-Coordination Compounds- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 8- Transition and Inner transition Elements – Online Notes
Next Chapter : Chapter-10-Halogen Derivatives– Online Notes
We reply to valid query.