Chemical Thermodynamics
Maharashtra Board-Class-12-Chemistry-Chapter-4
Notes-Part-2
Topics to be Learn : Part-2
|
Enthalpy (H) :
Enthalpy (H) : It is defined as the total energy of a system consisting of internal energy (U) and pressure-volume (P x V) type of energy, i.e. enthalpy represents the sum of internal energy (U) and product PV energy. It is denoted by H and is represented as
H = U + PV
Explanation :
- Enthalpy represents total heat content of the system.
- Enthalpy is a thermodynamic state function
- Enthalpy is an extensive property.
- The absorption of heat by a system increases its enthalpy. Hence enthalpy is called heat content of the system.
Change in enthalpy- ΔH :
Change in enthalpy, ΔH, is also state function given by
ΔH = H2 - H1 …(1)
where H1 and H2 are the enthalpies of initial and final states, respectively.
Consider a process in which a state of a system changes from an initial state A to a final state B.
Let H1, U1, P1, V1 and H2, U2, P2, V2 be the state functions of the system in initial and final states .
Then,
H1 = U1 + P1V1 and H2 = U2 + P2V2
From eq.(1)
ΔH = (U2 + P2V2) — (U1 + P1V1)
= (U2 — U1) + (P2V2 — P1V1)
= ΔU + ΔPV
where ΔU = U2 — U1
At constant pressure, P1 = P2 = P
P2V2 — P1V1= PV2 — PV1
= P (V2 — V1)
= P x ΔV
Hence, ΔH = ΔU+ PΔV
This is a relation for enthalpy change
Q. Show that the heat absorbed at constant pressure is equal to the change in enthalpy of the system.
Ans. By the first law of thermodynamics,
ΔU = Q + W
where ΔU is the change in internal energy
Q is heat supplied to the system
W is the work obtained.
Q = ΔU − W
If Q, is the heat absorbed at constant pressure by the system, so that the volume changes by ΔV against constant pressure P then,
W = − PΔV
QP = ΔU − (−PΔV)
QP = ΔU + PΔV ...(1)
If ΔH is the enthalpy change for the system, then
ΔH = ΔU + PΔV ……(2)
By comparing above equations, (1) and (2), we can write, Q1, = ΔH
Hence heat absorbed at constant pressure is equal to the enthalpy change for the system.
Since by increase in enthalpy heat content of the system increases, enthalpy is also called as the heat content of the system.
Relationship between ΔH and ΔU for chemical reactions :
- For any thermodynamic process or a chemical reaction at constant volume, ΔV = 0.
- Since ΔH = ΔU + PΔV, at constant volume ΔH = Δ U.
- In the reactions, involving only solids and liquids, ΔV is negligibly small, hence ΔH = ΔU.
- In a chemical reaction, in which number of moles of gaseous reactants and gaseous products are equal, then change in number of moles, Δn = n2 − n1= 0
- Since ΔH = ΔU + ΔnRT, as Δn = 0, ΔH = ΔU.
Relationship between ΔH and ΔU for gas phase reactions :
Consider a reaction in which n1 moles of gaseous reactant in initial state change to n2 moles of gaseous product in the final state.
Let H1, U1, P1, V1 and H2, U2, P2, V2 represent enthalpies, internal energies, pressures and volumes in the initial and final states respectively then,
n1A(g) → n2B(g)
The heat of reaction is given by enthalpy change ΔH as,
ΔH = H2 — H1
By definition, H = U + PV
∴ H1 = U1 + P1V1 and H2 = U2 + P2V2
∴ ΔH = (U2 + P2V2) — (U1 + P1V1)
= (U2 — U1) + (P2V2 — P1V1)
Now, ΔU= U2 — U1
Since PV= nRT,
For initial state, P1V1= n1RT
For final state, P2V2= n2RT
∴ P2V2 — P1V1= n2RT— n1RT
= (n2 — n1) RT = ΔnRT
Where Δn = [Number of moles of gaseous products] — [Number of moles of gaseous reactants]
ΔH = ΔU + ΔnRT
If QP, and QV, are the heats involved in the reaction at constant pressure and constant volume respectively, then since QP = ΔH and QV = ΔU.
∴ QP = QV + ΔnRT
Expression for work done in a chemical reaction :
Consider n1 moles of gaseous reactants A of volume V1 change to n2 moles of gaseous products B of volume V2 at temperature T and pressure P.
In the initial state, PV1 = n1RT
In the final state, PV2 = n2RT
PV2 — PV1 = n2RT— n1RT = (n2 — n1)RT = ΔnRT
where Δn is the change in number of moles of gaseous products and gaseous reactants.
Due to net changes in gaseous moles, there arises change in volume against constant pressure resulting in mechanical work, — PΔV.
W = —PΔV= —P (V2— V1) = —ΔnRT
Meaning of each term :
- If n1 = n2, Δn = 0, W = 0. No work is performed.
- If n2 > n1, Δn > 0, there is a work of expansion by the system and W is negative.
- If n2 < n1, Δn< O, there is a work of compression, hence work is done on the system and W is positive.
Q. Under what conditions ΔH = ΔU?
Ans. (i) ΔH = ΔU + PΔV, when ΔV = 0, ΔH = ΔU
(ii) ΔH = ΔU + ΔnRT, when Δn = 0, ΔH = ΔU
Enthalpies of physical transformations :
Enthalpy of phase transition : In phase transition, one phase of a substance is converted into another at constant temperature and pressure without change in chemical composition.
- During phase transformation, both the phases exist at equilibrium.
Solid ⇌ Liquid; Liquid ⇌ Vapour.
Different types of phase transitions :
The following are the types of phase changes :
Fusion : This involves the change of a matter from solid state to liquid state. In this heat is absorbed, hence it is endothermic (ΔH > 0).
H2O(g) → H2O(l)
Vaporisation or evaporation : This involves the change of a matter from liquid state to gaseous state.
In this heat is absorbed, hence it is endothermic (ΔH > 0).
H2O(l) → H2O(g)
Sublimation : This involves the change of matter from solid state directly into gaseous state. In this heat is absorbed, hence it is endothermic (ΔH > 0).
Camphor(s) → Camphor(g)
Enthalpy of fusion (ΔfusH) : The enthalpy change that accompanies the fusion of one mole of a solid into a liquid at constant temperature and pressure is called enthalpy of fusion.
For example,
H2O(s) \(\underrightarrow{\text{1 atm, 273K}}\) H2O(l), ΔfusH = 6.01 kJ mol−1
This equation describes that when one mole of ice melts (fuses) at 0°C (273 K) and 1 atmosphere, 6.01 kJ of heat will be absorbed.
Enthalpy of freezing (ΔfreezH) : The enthalpy change that accompanies the solidification of one mole of a liquid into solid at constant temperature and pressure is called enthalpy of freezing.
For example,
H2O(l) \(\underrightarrow{\text{1 atm, 273K}}\) H2O(s), ΔfreezH = −6.01 kJ mol−1
This equation describes that when one mole of water freezes (solidifies) at 0 °C (273 K) and 1 atmosphere, 6.01 kJ of heat will be released to the surroundings.
Enthalpy of vaporisation (ΔvapH) : The enthalpy change that accompanies the vaporisation of one mole of a liquid at constant temperature and pressure is called heat of vaporisation or evaporation.
For example,
H2O(l) \(\underrightarrow{\text{1 atm, 373K}}\) H2O(g), ΔvapH = + 40.7 kJ mol−1
This equation describes that when one mole of water is evaporated at 100 °C (373 K) and 1 atmosphere, 40.7 kJ of heat will be absorbed.
Enthalpy of sublimation (ΔsubH) : The enthalpy change or the amount of heat absorbed that accompanies the sublimation of one mole of a solid directly into its vapour at constant temperature and pressure is called enthalpy of sublimation. -
For example,
CO2(s) \(\underrightarrow{\text{1 atm, 195K}}\) CO2(g), ΔsubH = 25.2 kJ mol−1
This equation describes that when 1 mole of dry solid carbon dioxide, CO2(s) sublimes forming gaseous CO2 (g), 25.2 kJ of heat will be absorbed.
Process of sublimation :
The sublimation involves the conversion of a solid into vapour at constant temperature and pressure
For example,
H2O(s) → H2O(g), ΔsubH = 51.08 kJ mol−1 at 0 0C
This conversion takes place in two steps, first melting of solid into liquid and second vaporization of the liquid.
H2O(s)\(\underrightarrow{0^0C}\) H2O(l), ΔfusH = 6.01 kJ mol−1
H2O(l) \(\underrightarrow{0^0C}\) H2O(v), ΔvapH = 45.07 kJ mol−1
---------------------------------------------------
H2O(s) \(\underrightarrow{0^0C}\) H2O(g), ΔsubH = 51.08 kJ mol−1
It follows that
ΔsubH = ΔfusH + ΔvapH (See Fig.)

The increasing order of enthalpy of the given substance will be,
(s) < (l) < (g) '
This is because the conversion of H2O(s) to H2O(l) and further to H2O(g) involves absorption of heat.
Enthalpy of atomisation (ΔatoH) : The enthalpy change or amount of heat absorbed accompanying the dissociation of the molecules in one mole of a gaseous substance into free gaseous atoms at constant temperature and pressure is called enthalpy of atomization.
For example,
Cl2(g) → 2Cl(g) ΔatoH = 242 kJ mol−1
CH4(g) → C(g) + 4H(g) ΔatoH = 1660 kJ mol−1
Enthalpy of ionisation (ΔionH) : The enthalpy change or amount of heat absorbed accompanying the removal of one electron from each atom or ion in one mole of gaseous atoms or ions is called enthalpy of ionisation.
For example,
Na(g) → Na+(g) + e− ΔionH = 494 kJ mol−1
This equation describes that when one mole of gaseous sodium atoms, Na(g) are ionised forming gaseous ions, Na+(g), the energy required is 494 kJ.
Electron gain enthalpy (ΔegH) : It is defined as the enthalpy change, when mole of gaseous atoms of an element accept electrons to form gaseous ion.
E.g.
Cl(g) + e− → Cl(g) ΔegH = −349 kJ mol−1
It is the reverse of ionisation process.
Enthalpy of solution (ΔsolH): It is defined as the enthalpy change in a process when one mole of a substance is dissolved in specified amount of a solvent.
NaCl(s) + aq ⇌ NaCl(aq) ASMHH = 4 kJ 1'n01_1 I
Enthalpy of solution at infinite dilution (ΔsolH) : It is defined as the enthalpy change when one mole of a substance is dissolved in a large excess of a solvent, so that further dilution will not change the enthalpy at constant temperature and pressure.
For example,
HCl(g) + aq → HCl(aq), ΔsolH = − 75.14 kJ mol−1 .
Enthalpy of solution of an ionic compound :
An ionic compound dissolves in a polar solvent like water in two steps as follows :
Step-I : The ions are separated from the molecule involving crystal lattice enthalpy ΔLH.
MX(s) → M+(g) + X−(g)
ΔLH is always positive.
Step-II : The gaseous ions are hydrated with water molecules involving hydration energy, ΔhydH.
M+(g) + xH2O(l) → [M(H2O)x] +
X(g) +yH2O(l) → [X(H2O)y] −
ΔhydH is always negative.
The enthalpy change ΔsolH of solution is given by,
ΔsolH = ΔLH + ΔhydH
For example, consider enthalpy of solution of NaCl(s).
ΔLHNaCl = 790 kJ mol−1 .
ΔhydHNaCl = −786 kJ mol−1 .
Hence enthalpy change for solution of NaCl(S) is,
ΔsolH = ΔLHNaCl + ΔhydHNaCl
= 790 + (− 786)
= + 4 kJ mol−1 .
Therefore dissolution of NaCl in water is an endothermic process.
Thermochemistry :
Thermochemistry deals with heat changes or enthalpy changes in chemical reactions.
Explanation : Consider a reaction, Reactants —> Products
The heat changes ΔH for the reaction may be represented as,
ΔHreaction = ∑ Hproducts − ∑ Hreactants
where H represents enthalpy.
The energy released or absorbed during a chemical change appears in the form of heat energy
Enthalpy of chemical reaction (ΔrH) :
The enthalpy of a chemical reaction is the difference between the sum of the enthalpies of products and that of the reactants with every substance in a definite physical state and in the amounts (moles) represented by the coefficients in the balanced equation.
Explanation: Consider the following general reaction,
aA + bB —> cC + dD
The heat of the reaction ΔrH is the difference in sum of enthalpies of products and sum of enthalpies of reactants.
∴ ΔrH = ∑ Hproducts − ∑ Hreactants
= [cHC + dHD] − [aHA + bHB]
= ∑PH − ∑RH
where H represents enthalpy of the substance.
For endothermic reaction, ΔrH is positive, (ΔrH > 0)
For exothermic reaction, ΔrH is negative. (ΔrH < 0)
For example,
- N2(g) + O2(g) → 2NO2(g),
- ΔrH = 66.4 kJ (endothermic)
- 2KClO3(s) → 2KCl(s) + 3O2(g),
- ΔrH = −78 kJ (exothermic)
Endothermic process : A process taking place with the absorption of heat (from the surroundings) is called endothermic process.
For this process, Q is + ve, ΔrH + ve
- In this process products are less stable than reactants.
- This reaction requires supply of thermal energy.
Exothermic process : A process taking place with the evolution of heat is called exothermic process.
For this process, Q is − ve, ΔrH – ve
- In this process products are more stable than reactants.
- This reaction does not requires supply of thermal energy.
Sign convention used for ΔrH :
The change in enthalpy or heat of reaction ΔrH is given by
Sum of enthalpies
ΔrH = [Sum of enthalpies of products ] − [Sum of enthalpies of reactants ]
= ∑ Hproducts − ∑ Hreactants
(i) If the sum of enthalpies of products, ∑PH and reactants, ∑RH are equal then ΔrH for the reaction is zero, (ΔrH = 0).
i.e. ∑PH = ∑RH
∴ ΔrH = ∑PH − ∑RH =0
(ii) If the sum of enthalpies of products ∑PH is greater than the sum of enthalpies of reactants ∑RH, then ΔrH is positive, (ΔrH > 0). Since such reactions take place with the absorption of heat from surroundings, given by, they are called endothermic reactions.
∴ ∑PH > ∑ Hreactants
ΔrH > 0
(iii) If the sum of enthalpies of products ∑PH is less than the sum of enthalpies of reactants, ∑RH then ΔrH is negative, (ΔrH < 0). Since in such reactions heat is given out to the surroundings, they are called exothermic reactions.
∴ ∑PH < ∑ Hreactants
ΔrH < 0
Standard enthalpy of reaction (ΔrH0) :
Standard state of an element : It is defined as the most stable state of an element at 298 K and 1 atmosphere (or 1 bar).
In this state, the enthalpy of the element is assumed to be zero.
∴ H0element or in general Helement = 0
E.g. H°graphite = 0; (g) = 0; HNa(s) = 0; HHg(l) = 0
Thermochemical equation : It is defined as a balanced chemical equation along with the corresponding heat of reaction (ΔH) and physical states and number of moles of all reactants and all products appropriately mentioned.
E.g. C6H12O6(s) + 6O2(s) = 6CO2(g) + 6H2O(l)
ΔH = −2808 kJ mol−1
Guidelines for writing thermochemical equations :
(i) Reaction is represented by balanced chemical equation for the number of moles of the reactants and the products. E. g. CH4(g) + 2O2(g) = CO2(g) + 2H2O(l)
ΔrH0 = − 890 kJ mol−1
(ii) The physical states of all the substances in the reaction must be mentioned. E.g. (s) for solid, (L) for liquid and (g) for gas.
(iii) Heat or enthalpy changes are measured at 298 K and 1 atmosphere (or 1 bar).
(iv) ΔH0 is written at right hand side of thermochemical equation.
(v) Proper sign must be indicated for ΔH0. For endothermic reaction ΔH0 is positive, ( + ΔH0) and for exothermic reaction ΔH0 is negative, (− ΔH0).
(vi) The enthalpy of the elements in their standard states is taken as zero. (H0element = 0; H°C(s) = 0, (g) = 0
(vii) When all the substances taking part in the reaction are in their standard states, the enthalpy change is written as ΔH0.
(viii) The enthalpy of any compound is equal to its heat of formation.
(ix) In case of elements, the allotropic form must be mentioned. E.g. C(graphite), S(rhombic), Sn(white).
(x) For the reverse reaction, ΔH0 value has equal magnitude but opposite sign.
Q.Given the thermochemical equation, C2H2(g)+ 5/2 O2(g) → 2CO2(g)+ H2O(l), ΔrH0 = −1300 kJ
Write thermochemical equations when
(i) Coefficients of substances are multiplied by 2.
(ii) equation is reversed.
(i) 2C2H2(g)+ 5O2(g) → 4CO2(g)+ 2H2O(l),
ΔrH0 = 2 x −1300 kJ = −2600 kJ
(ii) 2CO2(g)+ H2O(l) → C2H2(g)+ 5/2 O2(g)
ΔrH0 = +1300 kJ
Standard enthalpy of reaction : It is defined as the difference between the sum of enthalpies of products and that of the reactants with every substance in its standard state at constant temperature (298 K) and pressure (1 atm).
Reactants → Products
ΔrH°reaction = ∑H°products − ∑H°reactants
Standard enthalpy of formation or standard heat of formation (ΔfH°) : It is defined as the enthalpy change ΔH° when one mole of a pure compound is formed in its standard state from its constituent elements in their standard states at constant temperature (298 K) and pressure (1 atmosphere or 1 bar). It is denoted by ΔfH°. E.g.
C(s) + O2(g) = CO2(g) ΔfH° = − 394 kJ mol−1 (ΔfH° may be positive or negative.)
Standard enthalpy of combustion or standard heat of combustion : It is defined as the enthalpy change when one mole of a substance in the standard state undergoes complete combustion in a sufficient amount of oxygen at constant temperature (298 K) and pressure (1 atmosphere or 1 bar). It is denoted by ΔCH°. p
E.g. C2H2(g)+ 5/2 O2(g) → 2CO2(g)+ H2O(l),
ΔCH0 = −1300 kJ mol−1 , (ΔCH0 is always negative.)
In the above reaction, the standard enthalpy change of the oxidation reaction, −1300 kJ is the standard enthalpy of combustion of C2H2(g).
| Calorific value : It is the enthalpy change or amount of heat liberated when one gram of a substance undergoes combustion.
. Calorific value = \(\frac{Δ_fH^0}{\text{ molar mass}}\) |
Q. Show that the standard heat of formation of a compound is equal to its enthalpy. (2 marks)
Answer : Consider the formation of one mole of gaseous CO2 in the standard state at 298 K and 1 atmosphere.
The thermochemical equation for formation can be represented as,
C(s) + O2(g) = CO2(g) ΔfH°= −394 kJ mol−1
Now heat of this reaction, ΔH° is,
ΔH°reaction = ∑PH0 − ∑RH0
∴ ΔfH°CO2(g) = H0CO2(g) – [H0C(s) + H0CO2(g)]
Since the enthalpies of elements in their standard states are zero,
H0C(s) = 0, H0CO2(g) = 0
∴ ΔfH°CO2(g) = H0CO2(g) – [0 + 0]
∴ ΔfH°CO2(g) = H0CO2(g)
Therefore standard heat of formation of a compound is equal to its enthalpy.
Standard enthalpy of reaction from standard enthalpies of formation :
The standard enthalpies of formation, ΔfH° of the compounds can be used to determine the standard enthalpy of reaction (ΔrH°).
ΔrH° of a reaction can be obtained by subtracting the sum of ΔfH° values of all the reactants from the sum of ΔfH° values of all the products with each ΔfH° value multiplied by the appropriate coefficient of that substance in the balanced thermo-chemical equation.
Consider following reaction :
aA + bB ——> cC + dD
The standard enthalpy of the reaction is given by,
ΔH°= [ Sum ΔfH° of the products] − [ Sum ΔfH° of the reactants]
= ∑ ΔfH°products — ∑ ΔfH°reactants
= [cΔfH°(C) + dΔfH°(D)] — [a ΔfH°(A) + b ΔfH°(B)]
where a, b, c and d are the coefficients (moles) of the substances A, B, C and D respectively.
Know This :
|
Bond enthalpy (or Bond energy) : The enthalpy change or amount of heat energy required to break one mole of particular covalent bonds of gaseous molecules forming free gaseous atoms or radicals at constant temperature (298 K) and pressure (1 atmosphere) is called bond enthalpy or bond energy.
For example, bond enthalpy of H2 is 436.4 kJ mol−1.
Bond enthalpy of diatomic molecules :
In case of diatomic molecules, since there is only one bond, the bond enthalpy is equal to heat of atomisation.
For example, heat of atomisation of HCl(g) is 431.9 kJ mol−1.
HCl(g) —> H(g) + Cl(g), ΔatmH° = 431.9 kJ mol−1.
∴ Bond enthalpy of HCl,
ΔatmH° = \(\frac{Δ_{atm}H}{\text{number of bonds broken}}\)
DH—Cl = 431.9/1 = 431.9 kJ mol−1. … (Bond enthalpy is generally denoted by D).
Bond enthalpy in polyatomic molecules :
- Each covalent bond in polyatomic molecules is associated with its own specific bond enthalpy.
- For polyatomic molecules the average bond enthalpy of a particular bond would be considered.
Consider bond enthalpy in H2O. The thermochemical equation for dissociation of H2O(g) is,
H2O(g) —> 2H(g) + O(g), ΔrH° = 927 kJ mol−1
In this, two O — H bonds are broken. It can be represented in stepwise as follows :
H2O(g) —> OH(g) + H(g) ΔH0HO-H = 499 kJ
OH(g) —> O(g) + H(g) ΔH0HO-H = 428 kJ
_____________________________________________________
H2O(g) —> O(g) + 2H(g) ΔrH° = 927 kJ
In above, even if two identical O — H bonds are broken, the energies required to break each bond are different.
The average bond enthalpy of O — H bond is,
ΔrH° = 927/2 = 463.5 kJ mol−1
Reaction and bond enthalpies :
- In a chemical reaction bonds are broken and formed.
- The enthalpies of reactions involving substances having covalent bonds are calculated by knowing the bond enthalpies of reactants and those in products.
- The calculations assume all the bonds of a given type are identical.
- Energy is always required to break a chemical bond while energy is always released in the formation of the bond.
The enthalpy change of a gaseous reactions (ΔrH°) involving substances with covalent bonds can be calculated with the help of bond enthalpies of reactants and products. (In case of solids we need lattice energy or heat of sublimation while in case of liquids we need heat of evaporation.)
ΔH°(reaction) = [Sum of bond enthalpies of bonds broken of reactants] — [Sum of bond enthalpies of bonds formed of products]
= ∑ ΔH°(bonds broken) − ∑ ΔH°(bonds formes)
For example for a following reaction,
H2(g) + Cl2(g) → 2HCl(g) OR
H—H(g) + Cl—Cl(g) → 2H—Cl(g)
ΔH°(reaction) = [ΔH°H-H + ΔH°Cl-Cl] − 2[ΔH°H-Cl]
If the energy required to break the bonds of reacting molecules is more than the energy released in the bond formation of the products, then the reaction will be endothermic and ΔH° reaction will be positive.
On the other hand if the energy released in the bond formation of the products is more than the energy required to break the bonds of reacting molecules then the reaction will be exothermic and ΔH° reaction will be negative.
Hess’s law of constant heat summation :
The law states that, “Overall the enthalpy change for a reaction is equal to sum of enthalpy changes of individual steps in the reaction”.
OR
Heat of reaction is same whether it is carried out in one step or in several steps.
Explanation :
Consider the formation of CO2(g). .
In one step : C(g) + O2(g) → CO2(g) ΔH = − 394 kJ
In two steps : C(s) + 1/2 O2(g) → CO(g) ΔH1 = −83 kJ
CO(g) + 1/2 O2(g) → CO2(g) ΔH2 = −311 kJ
ΔH = ΔH1+ ΔH2
− 394 kJ = − 83kJ + (−311)kJ
Hess’s law treats thermochemical equations mathematically i.e., they can be added, subtracted or multiplied by numerical factors like algebraic equations.
Applications of Hess’s law :
The Hess's law has been useful to calculate the enthalpy changes for the reactions with their enthalpies being not known experimentally.
It is used,
- To calculate heat of formation, combustion, neutralisation, ionization, etc.
- To calculate the heat of reactions which may not take place normally or directly.
- To calculate heats of extremely slow or fast reactions.
- To calculate enthalpies of reactants and products.
Spontaneous (irreversible) processes :
Spontaneous process : It is defined as a process that takes place on its own or without the intervention of the external agency or influence.
Characteristics :
- It occurs on its own and doesn’t require external agency.
- It proceeds in one direction and can’t be completely reversed by external stimulant.
- These processes may be fast or slow.
- These processes proceed until an equilibrium is reached.
The examples of the spontaneous processes are as follows :
- Expansion of a gas or flow of a gas from higher pressure to low pressure or a flow of heat from higher temperature to lower temperature.
- All natural processes are spontaneous.
- Flow of heat from hotter body to colder body.
- Acid-base neutralisation is a spontaneous reaction.
- Ice melts spontaneously above 0 °C.
Energy and spontaneity :
The spontaneous process takes place in a direction in which energy of the system decreases.
For example,
- Neutralisation reaction between NaOH and HCl solution is exothermic with release of energy.
The spontaneous process also takes place with the increase in energy by absorbing heat.
For example,
- Melting of ice at 0 °C by absorption of heat
- Dissolution of NaCl : NaCl dissolves spontaneously in water with the absorbtion of heat from the surroundings.
NaCl(s) + aq → NaCl(aq) → Na+(aq) + Cl−(aq)
ΔH° = + 3.9 kJ mol−1
- NaCl is dissolved in water, it does not react with water to produce NaOH and HCl.
Entropy : Being a state function and thermodynamic function it is defined as entropy change (ΔS) of a system in a process which is equal to the amount of heat transferred in a reversible manner (Qrev) divided by the absolute temperature (T), at which the heat is absorbed. Thus,
Entropy change = \(\frac{\text{Heat transferred reversibly}}{\text{Absolute temperature of heat transfer}}\)
∴ ΔS = Qrev/T
SI units of entropy are JK−1 and c.g.s. units are cal K−1. It is also expressed in terms of entropy unit (e.u.). Hence 1 e.u. = 1 J K−1.
Entropy is a measure of disorder in the system Higher the disorder, more is entropy of the system
- The amount of heat added to a system at higher temperature causes less disorder than when the heat is added at lower temperature.
- Since disorder depends on the temperature at which heat is added, ΔS relates reciprocally to temperature.
- This can also be explained from equation, ΔS = Qrev/T
Order in a system :
- When the atoms, molecules or ions constituting the system are arranged in a perfect order then the system is said to be in order. For example, in the solid state, the constituent atoms, molecules orions are tightly placed at lattice points in the crystal lattice.
- When solid melts forming a liquid or when a liquid vaporises, the constituents are separated and are in random motion imparting maximum disorder.
- As energy of the system decreases order increases.
Disorder in a system :
Increase in entropy is a measure of disorder in the system.
Consider following process :
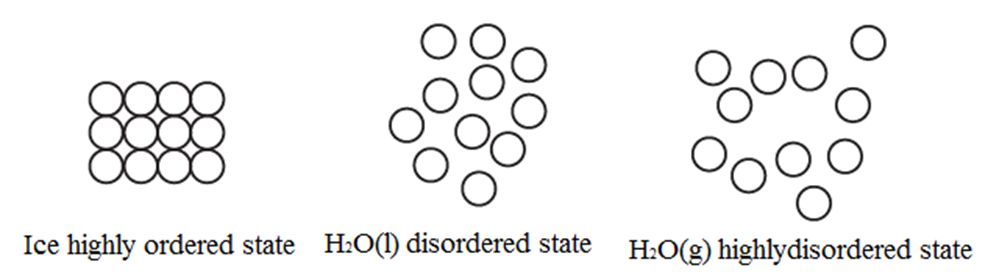
Change in order and entropy :
- The disorder or randomness is measured by entropy, denoted by S.
- Greater the disorder of a system larger is its entropy.
- Entropy of substance increases as it passes from solid to liquid to gas.
- The melting of ice and vaporisation of liquid water, in both processes entropy change ΔS > 0.
Look at the following processes :
(i) Dissolution of solid I2 in water :
For dissolution of solid I2,
I2(s) + aq → I2 (aq) ….. (ΔS > 0)
Ordered state disordered state
In the solid I2, there is ordered arrangement which collapses in solution increasing disorder and entropy, hence ΔS is positive.
(ii) In the dissociation of H2(g)
H2(g) → 2H(g) (ΔS > 0)
In the molecular state, two H atoms in every molecule are together but in atomic state the disorder is increased with the increase in entropy and hence ΔS > 0.
Q. State whether ΔS is positive, negative or zero for the following reaction.
(i) 2H2(g) + O2(g) → 2H2O(l)
(11) CaCO3(s) → CaO(s) + CO2(g)
(i) 2H2(g) + O2(g) → 2H2O(l)
Since the system is converted from gaseous state to a liquid state, the disorder is decreased, hence ΔS < O (negative).
(ii) CaCO3(s) → CaO(s) + CO2(g)
Since molecules of solid CaCO3 break giving gaseous CO2, disorder is increased hence ΔS > O (positive).
Q. How does entropy change in the following processes ? Explain.
(a) freezing of a liquid
(b) sublimation of a solid
(c) dissolving sugar in water
(d) condensation of vapour.
(a) Freezing of a liquid results in decrease in randomness and disorder, hence entropy decreases, ΔS < O.
(b) Sublimation of a solid converts it into vapour where the molecules or atoms are free to move randomly Hence disorder increases accompanying increase in entropy, ΔS > 0.
(c) Dissolving sugar in water separates the molecules of sugar in the solution increasing disorder and entropy, ΔS > 0.
(d) Condensation of vapour decreases the disorder and randomness, hence entropy, ΔS < 0.
Second law of thermodynamics in terms of entropy :
The second law of thermodynamics states that the total entropy of the system and its surroundings (universe) increases in a spontaneous process.
OR
Since all the natural processes are spontaneous, entropy of the universe increases.
It is expressed mathematically as
ΔStotal = ΔSsystem + ΔSsurr > 0 (for spontaneous process)
ΔSuniverse = ΔSsystem + ΔSsurr > 0
The total entropy increases during a spontaneous process that finally reaches equilibrium.
Entropy change for:
- ΔStotal, > 0, the process is spontaneous
- ΔStotal < 0, the process is non-spontaneous
- ΔStotal = O, the process is at equilibrium
Gibbs energy :
Gibbs free energy, G is defined as,
G = H — TS
where H is the enthalpy, S is the entropy of the system at absolute temperature T.
Since H, T and S are state functions, G is a state function and a thermodynamic function.
At constant temperature and pressure, change in free energy ΔG for the system is represented as,
ΔG = ΔH — T ΔS
Free energy = Total enthalpy —Temperature x Entropy change ' change change
This is called Gibbs free energy equation for ΔG.
In this ΔS is total entropy change, i.e., ΔSTotal.
The SI units of ΔG are J or kJ (or J mol” or kJ mol−1 ).
The c.g.s. units of ΔG are cal or kcal (or cal mol−1 or kcal mol−1)
Gibbs free energy and spontaneity of the process :
The total entropy change for a system and its surroundings accompanying a process is given by,
ΔStotal = ΔSsystem + ΔSsurr
By second law, for a spontaneous process, ΔStotal > O. lf + ΔH is the enthalpy change (or enthalpy increase) for the process, or a reaction at constant temperature (T) and pressure, then enthalpy change (or enthalpy decrease) for the surroundings will be −ΔH.
∴ ΔSsurr = −ΔH/T
∴ ΔStotal = ΔSsystem + ΔSsurr
∴ ΔStotal = ΔSsystem + (−ΔH/T)
∴ TΔStotal = TΔSsystem − ΔH
∴ TΔStotal = TΔSsystem − ΔH
∴ − TΔStotal = ΔH − TΔSsystem
By Gibbs equation,
ΔG = ΔH — T ΔS
By comparing above two equations,
ΔG = — TΔStotal
As ΔStotal increases, ΔG decreases.
For a spontaneous process, ΔStotal > 0
which is according to second law of themiodynamics.
∴ ΔG < 0.
Hence in a spontaneous process, Gibbs free energy decreases (ΔG < O) while entropy increases (ΔS > 0).
Therefore for a non-spontaneous process Gibbs free energy increases (ΔG > 0).
It can be concluded that for a process at equilibrium, ΔG = 0.
∴ The second law explains the conditions of spontaneity as below :
- ΔStotal > o and ΔG < 0, the process is spontaneous.
- ΔStotal < 0 and ΔG > 0, the process is non spontaneous.
- ΔStotal = 0 and ΔG = 0, the process is at equilibrium.
For a spontaneous or a non-spontaneous process,
ΔH and ΔS may be positive or negative (ΔH < 0 or ΔH > 0; ΔS < 0 or ΔS > 0). But ΔG must decrease, i.e., ΔG < O. If ΔG > 0, the process or a reaction will definitely be non-spontaneous.
This can be explained by Gibbs equation,
Δ G = ΔH — T ΔS.
Spontaneity of reactions when ΔH and ΔS are both negative : If ΔH and ΔS are both negative, then ΔG will be negative only when T ΔS < ΔH or when temperature T is low. Such reactions must be carried out at low temperatures.
Spontaneity of reactions when ΔH and ΔS are both positive : If ΔH and ΔS are both positive then ΔG will be negative if, T ΔS > ΔH; such reactions must be carried out at high temperature.
Spontaneity of reactions when ΔH is negative and ΔS is positive: If ΔH is negative (ΔH < 0) and ΔS is positive (ΔS > O) then for all temperatures ΔG will be negative and the reaction will be spontaneous. But as temperature increase, ΔG will be more negative, hence the reaction will be more spontaneous at higher temperature.
Spontaneity of reactions when ΔH is positive and ΔS is negative: If ΔH is positive, (ΔH > 0) and ΔS is negative (ΔS < O), ΔG will be always positive (ΔG > 0) and hence the reaction will be non-spontaneous at all temperatures.
Temperature condition for equilibrium :
For a system at equilibrium, free energy change ΔG is,
ΔG = ΔH — T ΔS
where ΔH is enthalpy change, ΔS is entropy change at temperature, T. Since ΔG = 0 at equilibrium,
0 = ΔH — T ΔS
TΔS = ΔH OR T = ΔH/ΔS
Hence at temperature T, changeover between forward spontaneous step and backward nonspontaneous step occurs and the system attains an equilibrium.
Here ΔH and ΔS are assumed to be independent of temperature.
Relationship between Gibb’s standard free energy change of the reaction and equilibrium constant K:
Consider following reversible reaction,
aA + bB ⇌ cC + dD
The reaction quotient Q is,
Q = \(\frac{[C]^c×[D]^d}{[A]^a×[B]^b}\)
The free energy change ΔG for the reaction is
ΔG = ΔG° + RT ln Q
Where ΔG° is the standard free energy change.
At equilibrium
Q = \(\frac{[C]^e_c×[D]^e_d}{[A]^e_a×[B]^e_b}\) =K
ΔG = ΔG° + RT ln K
at equilibrium ΔG = 0
0 = ΔG° + RT ln K
ΔG° = − RT ln K
ΔG° = − 2.303 RT log10K.
| Important Terms and Formulae :
W = − P (V2 − V1) = − PΔV(For expansion) W = P (V2 − V1) = P ΔV(For compression) Wmax = −2.303nRT1og10 \(\frac{V_2}{V_1}\) Wmax = −2.303 nRT log10 \(\frac{P_1}{P_2}\) ΔU = q+ W H = U + PV ΔH = ΔU + PΔV ΔH = ΔU + ΔnRT Hess’slaw: ΔH = ΔH1 + ΔH2 + ΔH3 ΔS = \(\frac{q_{rev}}{T}\) = \(\frac{ΔH}{T}\) G = H − TS ΔG = ΔH − TΔS ΔG° = − 2.303 RT log10K (i) ΔG = 0, the system is at equilibrium (ii) ΔG < 0, the process is spontaneous (iii) ΔG > 0, the process is non-spontaneous. |
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-3- Ionic Equilibria – Online Notes
Next Chapter : Chapter-5-Electrochemistry – Online Notes
All formulas wants
will be available soon