Aldehydes, Ketones and Carboxylic acids
Maharashtra Board-Class-12-Chemistry-Chapter-12
Notes-Part-2
Topics to be Learn : Part-2
|
Preparation of aldehydes and ketones :
General methods of preparation of aldehydes and ketones :
(i) By oxidation of alcohols :
(a) Aldehydes and ketones are prepared by the oxidation of primary and secondary alcohols respectively.
When a primary alcohol is oxidized with potassium dichromate and dil. H,SO, under controlled conditions, an aldehyde is obtained.
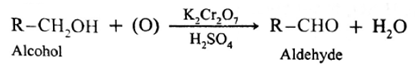
Example : When ethanol is oxidized with potassium dichromate and dil. H,SO, under controlled conditions, acetaldehyde (ethanal) is obtained.
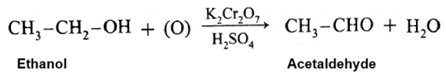
(b) By dehydrogenation of alcohols : This method has industrial application. Aldehydes and ketones are prepared by passing the vapours of primary and secondary alcohols respectively over hot copper powder.
When vapours of secondary alcohol is passed over heated copper at 573 K, dehydrogenation takes place, a ketone is obtained.
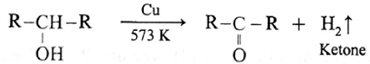
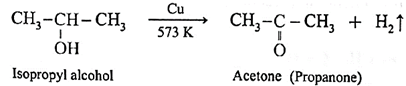
Example : When vapours of isopropyl alcohol is passed over heated copper at 573 K, acetone (propanone) is obtained.
(ii) From hydrocarbons :
(a) By ozonolysis : Alkene reacts with ozone to give ozonide which on decomposition with zinc dust and water gives aldehyde and/or ketones.
When a stream of ozonised oxygen is passed through a solution of an alkene, in organic solvent, an unstable addition cyclocompound, ozonide is formed which on reduction with zinc dust and water forms an aldehyde or a ketone or a mixture of both.
(i) Formaldehyde : Under these conditions ethylene gives formaldehyde.
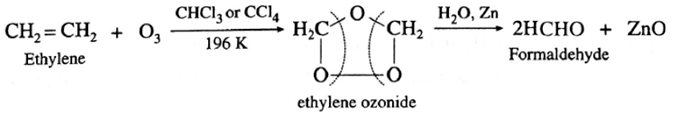
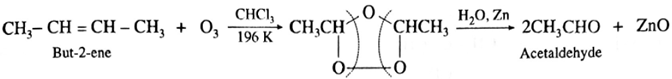
(ii) Acetaldehyde : Symmetrically disubstituted alkene like but-2-ene gives acetaldehyde.
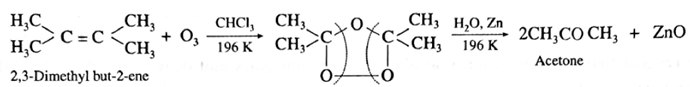
(iii) Acetone : Tetrasubstituted alkene like 2,3-dimethyl but-2-ene gives acetone.
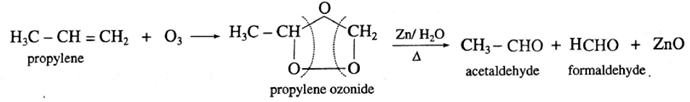
(iv) Propylene on reaction with ozonised oxygen in the organic solvent forms propylene ozonide which on reduction with zinc dust and water forms acetaldehyde and formaldehyde.
(b) By hydration of alkynes : Alkynes react with water in presence of 40% sulfuric acid and 1% mercuric sulfate to give aldehydes or ketones.
Examples :
Acetaldehyde : On passing acetylene through warm 40% H2SO4 in the presence of 1 % HgSO4, vinyl alcohol is obtained which tautomerises and forms acetaldehyde. It is a hydration reaction.
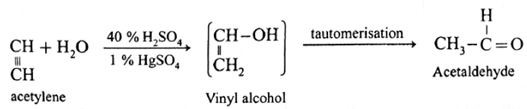
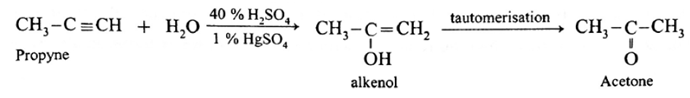
Acetone : On passing propyne through warm 40 % H2SO4 in the presence of 1 % HgSO4 alkenol is obtained which on tautomerisation form acetone.
Other methods of preparation of aldehydes and ketones :
Some methods of preparation of aldehydes and ketones involve common starting functional groups but different types.
(1) From acyl chlorides (Acid chlorides) :
Aldehydes and ketones both can be obtained from acyl chloride, but the reactions involved are different.
Examples :
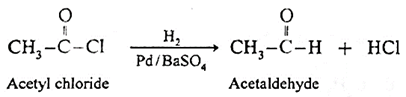
Preparation of aldehyde from acetyl chloride :
(i) Acetyl chloride is reduced to corresponding aldehyde by hydrogen using a palladium catalyst (Pd) poisoned with barium sulfate BaSO4. This reaction is known as Rosenmund reduction.
(ii) When benzoyl chloride is hydrogenated in the presence palladium on barium sulphate (Pd / BaSO4), benzaldehyde is obtained. This reaction is called Rosenmund reduction.
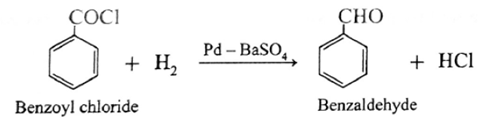
(2) Preparation of ketone (aliphatic and aromatic) from acyl chloride :
Preparation of aliphatic ketones from acyl chloride:
ketones are obtained from acyl chloride by reaction with dialkyl cadmium which is prepared by the treatment of cadmium chloride with Grignard reagent.
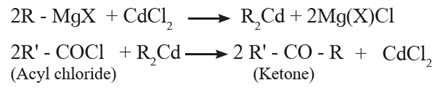
Examples :
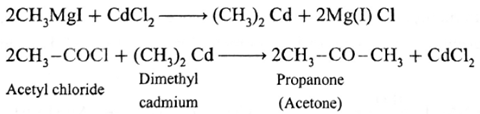
(i) Prepare Propanone (acetone) from Grignard reagent : Grignard reagent (methyl magnesium iodide) reacts with cadmium chloride to give dimethyl cadmium. When acetyl chloride reacts with dimethyl cadmium, propanone (acetone) is obtained.
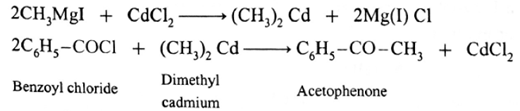
(ii) Prepare acetophenone from Grignard reagent : Grignard reagent (methyl magnesium iodide) reacts with cadmium chloride to give dimethyl cadmium. When benzoyl chloride reacts with dimethyl cadmium, acetophenone is obtained.
Preparation of aromatic ketones from acyl chloride :
Aromatic ketones are prepared by Friedel Craft's acylation reaction
(i) From nitriles : Aldehydes and ketones both can be obtained from nitriles but by different reaction.
Preparation of aldehydes from nitriles : Nitriles are reduced to imine hydrochloride by stannous chloride in presence of hydrochloric acid which on acid hydrolysis give corresponding aldehydes. This reaction is called Stephen reaction.

Examples :
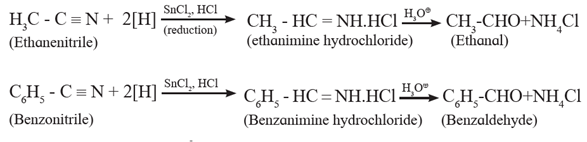
Alternatively, nitriles are selectively reduced by diisobutyl aluminium hydride (DIBAl-H) to imines which on acid hydrolysis to aldehydes.
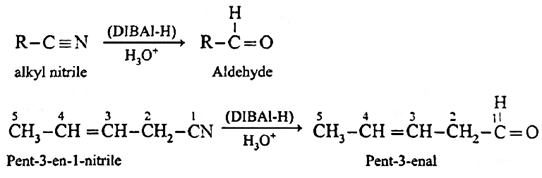
Preparation of Acetone (using Grignard reagent): Acetonitrile (ethanenitrile) reacts with methyl magnesium iodide in presence of dry ether to give imine complex which on hydrolysis gives acetone. During reaction acetonitrile and methyl magnesium iodide should be taken in equimolecular proportion.
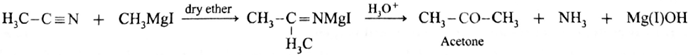
(3) From aromatic hydrocarbons :
Aromatic aldehydes and ketones are both prepared from aromatic hydrocarbons but by different methods.
Preparation of aromatic aldehydes from hydrocarbon :
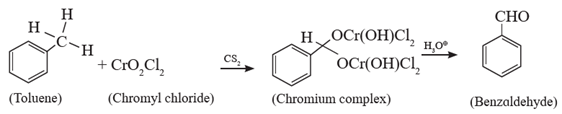
(i) Etard reaction :
Benzaldehyde from toluene. (Etard oxidation) : When toluene is treated with solution of chromyl chloride (CrO2Cl2) in Cs2, brown chromium complex is obtained, which on acid hydrolysis gives benzaldehyde. This reaction is known as Etard reaction.
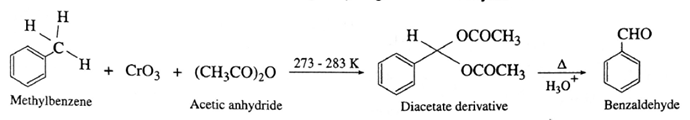
(ii) By oxidation of methyl arene using CrO3 :
Benzaldehyde from methyl arene : Methylarene is converted into a benzyllidene diacetate on treatment with chromium oxide in acetic anhydride at 273-278 K. The diacetate derivative on acid hydrolysis gives benzaldehyde.
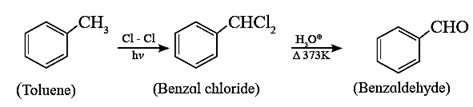
(iii) By side chain chlorination of toluene:
Side chain chlorination of toluene gives benzal chloride which on acid hydrolysis at 373K gives benzaldehyde. Benzaldehyde, is manufactured commercially by this method.
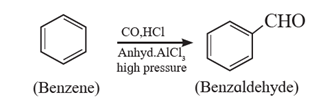
(iv) Gatterman –Koch formylation of arene:
When benzene is treated with vapours of carbon monoxide and hydrogen chloride in the presence of a catalyst mixture of AICI; and CuCl under high pressure, benzaldehyde is obtained. This reaction is called Gattermann-Koch synthesis.
Preparation of Aromatic ketones from hydrocarbons :
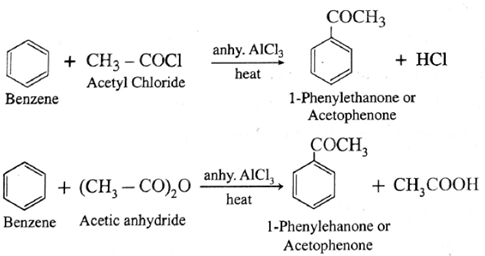
By Friedel-crafts acylation of arene : The reaction in which hydrogen atom of benzene is replaced by an acyl group in the presence of anhydrous AlCl3 is called Friedel-Craft’s acylation. When benzene is heated with an acetyl chloride or acetic
anhydride in the presence of anhydrous AlCl3 forms acetophenone (1-Phenyl ethanone).
Electrophile : R—C + = O acylium ion
Formation of the electrophile :
R-COCl + AlCl3 — R—C + = O + AlCl4—
(i) Preparation of acetophenone from benzene using (i) acetyl chloride (ii) acetic anhydride.
(ii)

Know This : The compounds which are used in preparation of benzophenone by Friedel—Crafts reaction are : (i) Benzene, (ii) Benzoyl chloride and (iii) Anhyd. aluminium chloride
Preparation of aldehydes only from esters : Aliphatic or aromatic esters are reduced to aldehydes by using diisobutyl aluminium hydride DIBAl-H or AlH (i-Bu)2. The reaction is usually carried out at 195 K to prevent further reduction of the aldehyde produced.
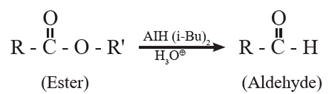
When ethyl propionate is reduced in presence of diisobutyl aluminium hydride (DIBAI-H), propionaldehyde is obtained.
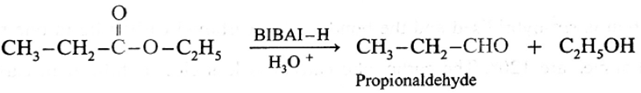
Preparation of carboxylic acids :
Structure of carboxyl group : In carboxyl group, the carboxyl carbon is sp2-hybridised and the bonds to the carboxyl carbon lie in one plane.
The C—C = O and O = C—O bond angles are 120°. The carboxylic carbon is less electrophilic than carbonyl carbon because of the resonance structures shown below :
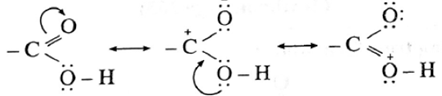
(1) Preparation of carboxylic acid from nitriles and amides :
Alkyl cyanides or alkyl nitriles on acid or alkaline hydrolysis give corresponding carboxylic acids.
Acid Hydrolysis of Alkyl cyanide : When alkyl cyanide is boiled with dilute mineral acid, it gives corresponding carboxylic acid. In this, acid amide is obtained as the intermediate product.
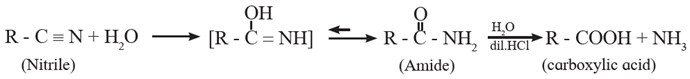
OR the final equation becomes
![]()
(i) When methyl cyanide is heated with dilute hydrochloric acid or dilute sulphuric acid, ethanoic acid is obtained.
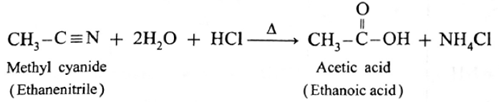
(ii) When ethyl cyanide (propionitrile) is boiled with dilute HCI or dilute H,SO,, propionic acid is obtained.
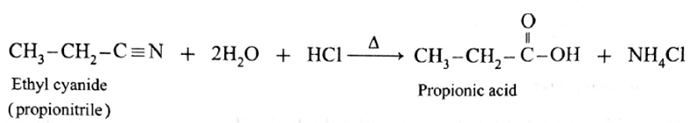
(iii) When benzamide is heated with dil. HCI, benzoic acid is obtained.
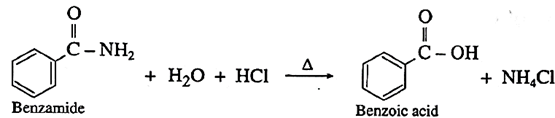
(2) Preparation of carboxylic acid from acyl chloride and anhydrides :
(a) Acyl chlorides on hydrolysis with water give carboxylic acids. This method is useful for preparation of aliphatic as well as aromatic acid. The reaction is carried out in presence of a base pyridine or NaOH to remove HCl generated.
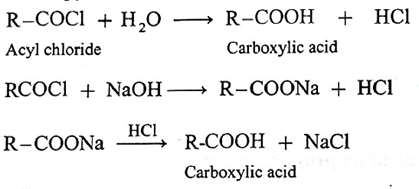
Acetyl chloride reacts with water almost explosively while benzoyl chloride very slowly.
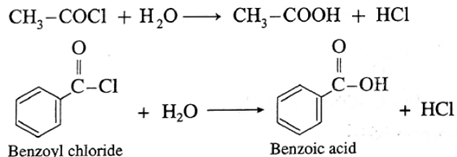
(b) Acid anhydrides react with water to give carboxylic acid.
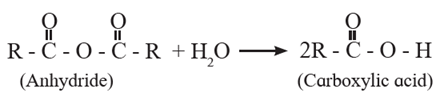
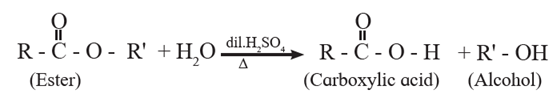
(3) Preparation of carboxylic acid from esters :
Carboxylic acids can be obtained from esters either by acid hydrolysis or alkaline hydrolysis.
(a) Acid hydrolysis of ester : Esters on hydrolysis with dilute mineral acid like dilute HCl or dilute H2SO4 give the corresponding carboxylic acid .
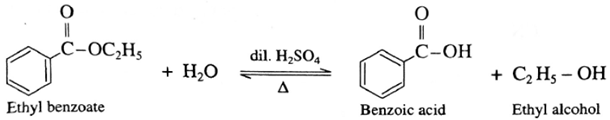
Example :
Benzoic acid from ethyl benzoate : When an ethyl benzoate is heated with dil. H2SO4 undergoes hydrolysis to form benzoic acid and ethyl alcohol.
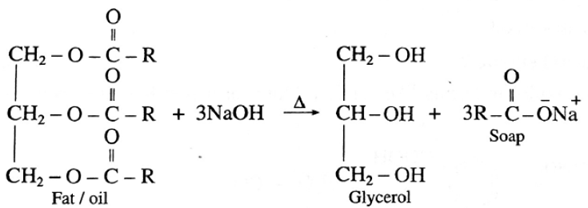
(b) Alkaline hydrolysis of ester using dilute alkali like dilute NaOH or dilute KOH form solution of water soluble sodium or potassium salt of the acid (carboxylate). On acidification with concentrated HCl, free acid is formed.

The sodium or potassium salts of higher fatty acids are known as soaps. Hence alkaline hydrolysis of esters is called saponification.
Soap preparation : When fat or oil is hydrolysed using sodium or potassium hydroxide solution, soap obtained remains in colloidal form. Soap and glycerol are separated by adding sodium chloride. Soap precipitates out due to common ion effect, and glycerol remains in the solution can be recovered by fractional distillation.
(4) Preparation of carboxylic acid from alkyl benzene :
Aromatic carboxylic acids can be prepared by oxidation of alkyl benzene with dilute HNO3 or alkaline /acidic KMnO4 or chromic acid. The entire 264 alkyl chain , regardless of its length, is oxidized to a carboxyl group. (Tertiary alkyl substituent on benzene, however, is not oxidized).
Examples :
(a)
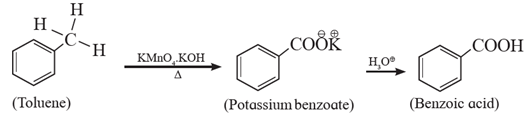
(b) Benzoic acid from styrene :
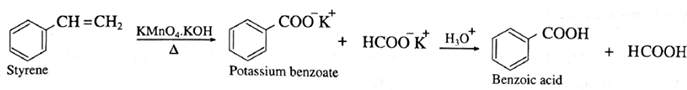
(5) Preparation of carboxylic acid from alkenes :
Carboxylic acids can also be prepared by the oxidation of alkenes by KMnO4 in dilute H2SO4.
Examples:
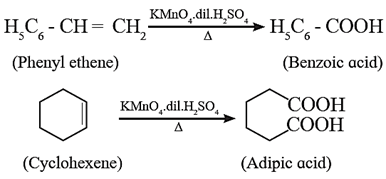
(6) Preparation of carboxylic acid from Grignard reagent :
Addition reaction of carbon dioxide (O = C = O) to Grignard reagent, forming a complex and further formation of carboxylic acid is called carbonation of Grignard reagent.

Example :
When methyl magnesium iodide is added to solid carbon dioxide, a complex is formed which on acid hydrolysis forms acetic acid.
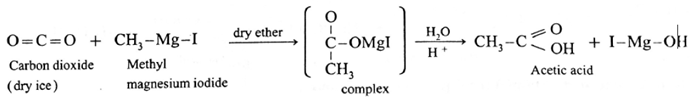
Physical properties :
(1) Nature of intermolecular forces :
In aldehydes and ketones :
- The carbonyl bond (>C=O) in aldehydes and ketones is a polar covalent bond. As a result, these compounds contain dipole-dipole forces of attraction. (Fig.)
- The molecules orient in such a way as to have oppositely polarized atoms facing each other.
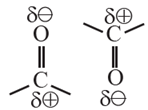
In Carboxyl group of carboxylic acid :
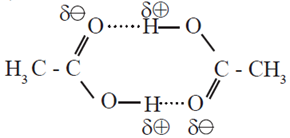
- Carboxyl group of carboxylic acid contains O-H bond which is responsible for formation of hydrogen bonding. Thus, carboxylic acids have the strongest intermolecular forces of attraction. (Fig.).
(2) Physical state and boiling points of aldehydes and ketones :
- Formaldehyde is a gas at room temperature and has irritating odour.
- Acetaldehdye is extremely volatile, colourless liquid. Higher aldehydes have pleasant odour.
- Acetone is a liquid at room temperature and has pleasant odour but most of the higher ketones have bland odours.
Boiling points of aldehydes and ketones :
Increasing boiling points in the homologous series of aldehydes and ketones are listed in below Table
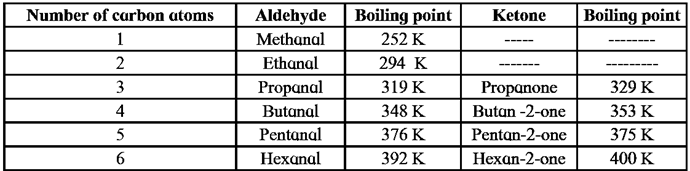
(3) Solubility of aldehydes and ketones :
The oxygen atom of ( >C=O) can involve in hydrogen bonding with water molecule (Fig). As a result of this, the lower aldehydes and ketones are water soluble. Example : acetaldehyde, acetone.
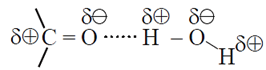
As the molecular mass increases, the proportion of hydrocarbon part of the molecule increases which cannot form hydrogen bond; and the water solubility decreases.
(4) Physical state, boiling points and solubilities of carboxylic acids :
Physical state : Lower aliphatic carboxylic acids upto nine carbon atoms are colourless liquids with irritating odours. The higher homologues are colourless, odourless wax like solids, have low volatility.
Boiling points :
- Carboxylic group (- COOH) in acids is highly polar. In liquid state, pair of carboxylic acid molecules is held together by two intermolecular hydrogen bonds, have higher aggregations and in the vapour.
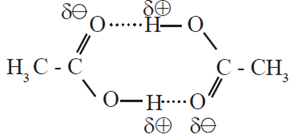
Dimer of acetic acid (Two molecules held by two hydrogen bonds)
- Intermolecular hydrogen bonding in carboxylic acids state most of the carboxylic acids cxist as dimmers in which two molecules are held by two hydrogen bonds.
- Acidic hydrogen of one molecule forms hydrogen bond with carbonyl oxygen of the other molecule. This doubles the size of the molecule resulting in increase in intermolecular van der Waals forces, which in turn results in high boiling points.
- Therefore, carboxylic acids possess higher boiling points than those of alcohols, aldchydes, ketones, ether, hydrocarbons of comparable molecular masses,
Boiling points of lower carboxylic acids :
| Name | Formula | Boiling point in K |
| Formic acid | HCOOH | 373 K |
| Acetic acid | CH3COOH | 391 K |
| Propionic acid | CH3CH2COOH | 414 K |
| Butyric acid | CH3CH2CH2COOH | 437 K |
| Valeric acid | CH3CH2CH2CH2COOH | 460 K |
Solubilities :
- Lower aliphatic carboxylic acids are miscible with water due to the formation hydrogen bonding with water molecules.
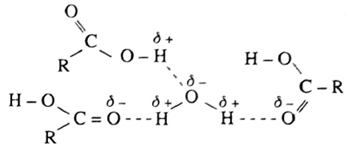
- Hydrogen bonding between acid and water. As the molecular mass increases the solubility of carboxylic acids in water decreases.
- The insolubility of carboxylic acids is due to increased hydrophobic interaction of hydrocarbon part with water.
Carboxylic acids are more acidic than phenols and alcohols :
Explanation :
(1) Carboxylic acid loses a proton as compared to phenol. Consider the ionization of carboxylic acid and phenol.

Due to delocalization the negative charge over the ortho and para positions of aromatic ring, phenoxide anion is more stable than phenol. Thus phenol easily undergoes ionization.
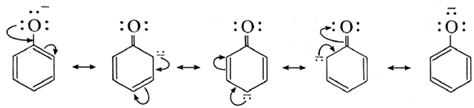
However, alcohol and alkoxide ion are single structures. In an alkoxide anion the negative charge is localized on a single oxygen atom. Hence, phenols are more acidic than alcohols.
(2) Carboxylic acid has two resonance hybride non equivalent structures (I & II) while carboxylate anion has two resonance hybrid equivalent structures (III & IV). The carboxylate ion is more stable than carboxylic acid and equilibrium is shifted towards the direction of increased ionization.
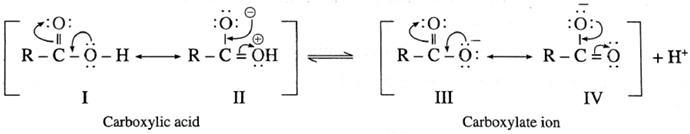
Carboxylate ion has two equivalent resonance structures with negative charge is delocalized over two electronegative oxygen atoms. Phenoxide anion has non-equivalent resonance structures in which negative charge is delocalized over one oxygen atom and less electronegative carbon atom. As a result carboxylate anion is more stable than phenoxide ion. Hence carboxylic acids ionize to the greater extent than phenol furnishing higher concentration of H+ ions. Therefore, carboxylic acids are more acidic than phenols and alcohols.
| Remember :
Relative strength of intermolecular force : H-Bond > dipole-dipole attraction > van der Waals force. Hence, Boiling points : Carboxylic acids > Alcohols > Ketones > Aldehydes > ether > Alkanes |
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 11- Alcohols, Phenols and Ethers – Online Notes
Next Chapter : Chapter-13-Amines– Online Notes
We reply to valid query.