Alcohols, Phenols and Ethers
Maharashtra Board-Class-12-Chemistry-Chapter-11
Notes-Part-2
Topics to be Learn : Part-2
|
Physical and Chemical Properties of alcohols and phenols :
Physical Properties of alcohols and phenols :
The properties of alcohols and phenols are mainly due to the hydroxyl group.
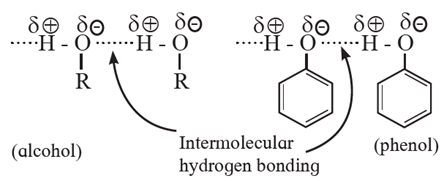
(i) Nature of intermolecular forces : Due to presence of —OH groups, alcohols and phenols are polar molecules, The polar —OH groups are held together by the strong intermolecular forces i.e. hydrogen bonding.
(ii) Physical State : Lower alcohols are colourless, toxic liquids having characteristic
alcoholic odour. Pure phenol is colourless, toxic, low melting solid having characteristic carbolic or phenolic odour.
(iii) Boiling Points : The boiling points of alcohols and phenols increase with increase in their molecular mass.
Ethanol has higher boiling point than ethane :
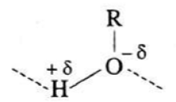
- The hydroxyl group in alcohols is highly polar. The H-atom has partial positive charge and the oxygen atom has partial —ve charge. The hydroxyl group in ethanol is extensively hydrogen bonded.
- Large number of ethanol molecules associated together by intermolecular hydrogen bonding. The energy required to separate the molecules by breaking hydrogen bond into vapour state is higher. This results in increasing the boiling point of ethanol.
- On the other hand, in ethane (alkane) there is no hydrogen bonding between the molecules. The molecules of ethane are held together by weak van der Waals forces of attraction. Hence, these molecules can be easily separated.
Thus, ethanol has higher boiling point than that of ethane.
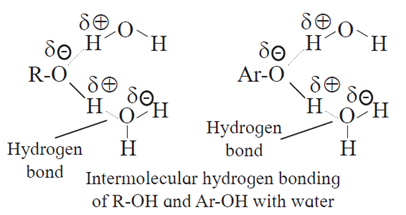
(iv) Solubility : Solubility of alcohols and phenols in water due to their ability to form intermolecular hydrogen bonding.
Phenols and lower alcohols (having upto three carbons) show appreciable solubility in water due to their ability to form intermolecular hydrogen bonding with water molecule.
Methanol is more soluble in water than propan-1-ol :
- Methanol being lower members of alcohols is more soluble in water, but as the size of an alkyl group or molecular weight of alcohol increases the solubility decreases.
- The solubility of methanol in water is due to polar characters of alcohols (R-O-δ–H+δ) and water ( H+δ-O-δ-H+δ).
- The solubility of methanol is due to the formation of intermolecular hydrogen bonding between polar molecules of methyl alcohol and water. Hence, methyl alcohol is a associated liquid.
- In methyl alcohol, size of methyl group being very small, —OH group constitutes major part of the molecule giving more solubility. As size of alkyl group increases, the non-polar character increases the solubility decreases.
Hence, methanol is more soluble in water than propan-1-ol.
Chemical properties of Alcohols and Phenols :
Laboratory test :
- Aqueous solution of alcohols and phenols can be tested with litmus paper.
- Aqueous solution of alcohols is neutral to litmus (neither blue nor red litmus change colour).
- Aqueous solutions of phenols turn blue litmus red. Thus, phenols have acidic character.
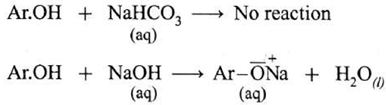
- Alcohol does not react with ag. NaHCO; or aq.NaOH
- Phenol does not react with ag. NaHCO, but reacts NaOH with NaOH
- Alcohol does not react with neutral ferric chloride.
- Phenol reacts with neutral ferric chloride solution to give deep (purple / violet / green) colouration of ferric phenoxide.
3Ar—OH + FeCl3 → (Ar—O)3Fe + 3HCl
(neutral) (deep colour)
- Alcohol is non-corrosive.
- Phenol is corrosive
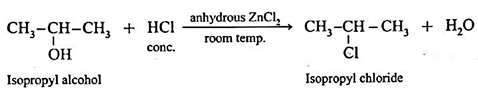
. Distinguishing test for alcohols (Lucas test) :
- Lucas reagent is a mixture of concentrated HCI and anhydrous ZnCl,. It is used to distinguish between primary, secondary and tertiary alcohols.
- Alcohols are soluble in Lucas reagent. Alcohol with Lucas reagent forms an alkyl chloride, R—Cl which is insoluble and gives cloudiness and forms separate layer.
- The time required for cloudiness to appear is based on the type of the alcohol and its reactivity :
R - OH \(\frac{HCl}{ZnCl_2}\)> R - Cl
(i) Primary alcohol : It reacts with the Lucas reagent very slowly and on heating forms alkyl chloride. The cloudiness and separation of a layer takes place after a long time on heating.

(ii) Secondary alcohol : It reacts with the Lucas reagent much faster to form alkyl chloride. The cloudiness and separation of layer takes place slowly.
(iii) Tertiary alcohol : It reacts immediately with the Lucas reagent at room temperature to form alkyl chloride. The cloudiness and separation of layer takes place instantaneously.

Reactions due to breaking of O -H bond :
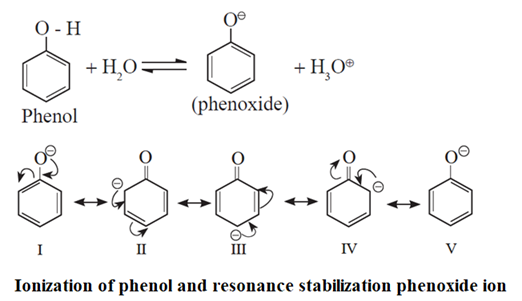
The reactivity of alcohols and phenols towards ionization of O-H bond in them is different. This difference lies in the extent of stabilization of their respective conjugate bases by electronic effects as shown below.
Ionization of alcohols :
![]()
The alkyl group's electron-donating inductive activity (+I effect) destabilises the alkoxide ion (the conjugate base of alcohol). As a result, alcohol does not ionise much in water and behaves in aqueous media like a neutral chemical.
Ionization of phenol :
Phenoxide ion, the conjugate base of phenol, is resonace stabilized by delocalization of the negative charge. Therefore phenol ionizes in aqueous medium to a moderate extent, and thereby shows a weak acidic character.
Question : What are the electronic effects exerted by -OCH3 and -Cl ? predict the acid strength of,
![]()
The electronic effects exerted by —Cl and —O CH3 are as follows :
- Cl being more electronegative atom it pulls the bonding electrons towards itself. This is known as negative inductive effect (—I).
- —OCH3 is less electronegative group which repels the bonding electrons away from it. This is known as positive inductive effect ( + I).
- The relative to parent phenol,
![]()
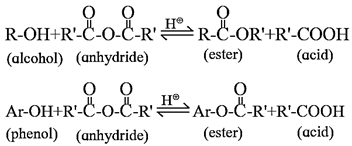
Esterification :
The reaction between alcohol or phenol with a carboxylic acid is carried out in the presence of concentrated sulphuric acid an ester is formed. This reaction is called esterification.
- The reaction is reversible and can be shifted in the forward direction by removing water as soon as it is formed.
- The formation of an ester is favoured using excess of alcohol in the presence of conc. H2SO4.

- Alcohols and phenols react with acid anhydrides in presence of acid catalyst to form ester.
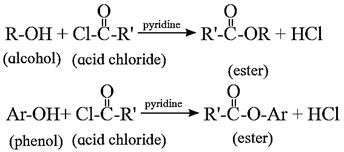
- The reaction of alcohol and phenols with acid chloride is carried out in the presence of pyridine (base), which neutralizes HCl.
Examples :
(i) Action of acetic acid on ethanol : When ethanol is treated with acetic acid in the presence of conc. sulphuric acid, ethyl acetate (ester) is formed.
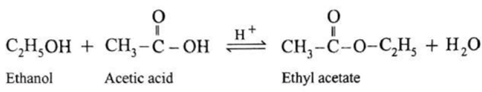
(ii) Action of acetic anhydride on ethanol : When ethanol is treated with acetic anhydride in the presence of conc. sulphuric acid, ethyl acetate (ester) is formed.
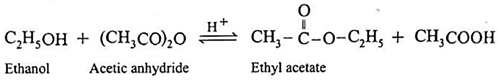
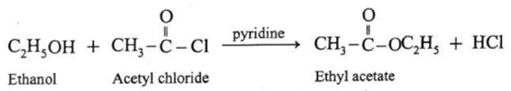
(iii) Action of acetyl chloride on ethanol : When ethanol is treated with acetyl chloride in the presence of pyridine, ethyl acetate (ester) is formed. (Pyridine neutralises HCl formed during reaction).
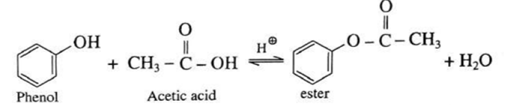
(iv) Action of acetic acid on phenol : When phenol is treated with acetic acid in the presence of conc. sulphuric acid, (ester) is formed.
(v) Action of acetic anhydride on phenol : When phenol is treated with acetic anhydride in the presence of conc. sulphuric acid, (ester) is formed.
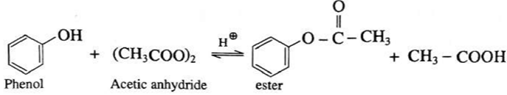
(vi) Action of acetyl chloride on phenol : When phenol is treated with acetyl chloride in the presence of conc. sulphuric acid, (ester) is formed.
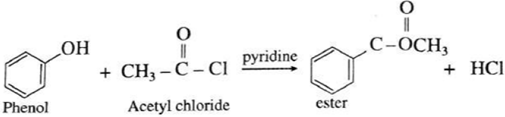
Acetyl derivatives : The CH3-CO- group is called acetyl group. The acetate esters of alcohols or phenols are also called ‘acetyl derivatives’ of alcohols or phenols respectively.
When acetic anhydride is treated with salicyclic acid in presence of glacial acetic acid, acetyl salicyclic acid (aspirin) is obtained.

Reaction due to breaking of C-O bond in alcohols :
(i) Reaction with hydrogen halides : Tertiary alcohols react rapidly with hydrogen halides; secondary alcohols react somewhat slower; and primary alcohols, even more slowly. The order of reactivity of hydrogen halides is HI > HBr > HCl. HCl reacts only in the presence of anhydrous ZnCl2. No catalyst is required in the case of HBr and HI.
When ethyl alcohol is heated with HBr, ethyl bromide is formed. (HBr is prepared in situ by adding NaBr to HCl or H2SO4)
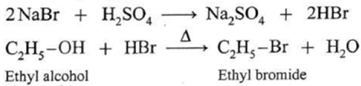
(ii) Reaction with phosphorous halide : Alcohols react with phosphorous pentahalide (PX5) and phosphorous trihalide (PX3) to form alkyl halides.
Ethanol : When ethanol is treated with PCl3, ethyl chloride is obtained.
3CH3CH2—OH + PCl3 → 3CH3—CH2Cl + H3PO3
Ethanol : When ethanol is treated with PCl5, ethyl chloride is obtained
CH3CH2—OH + PCl5 → CH3CH2Cl + POCl3 + HCl
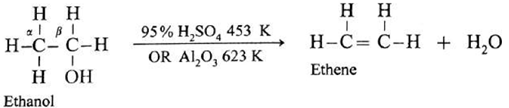
(iii) Dehydration of alcohols to alkenes : Removal of water from an alcohol is called dehydration of alcohol.
- Alcohol when treated with concentrated sulphuric acid or phosphoric acid or alumina undergoes dehydration to form alkene and water.
- The reaction gives more substituted alkene as the major product, in accordance with Saytzeff rule.
The ease of dehydration of alcohols is in the following order :
tert-alcohol (3°) > secondary (2°) > primary (1°)
(a) Primary (1°) alcohol is dehydrated by heating it with 95% H2SO4 at 453 K.
(b) Secondary alcohol (2°) is dehydrated by heating with 60% H2SO4 at 373 K.
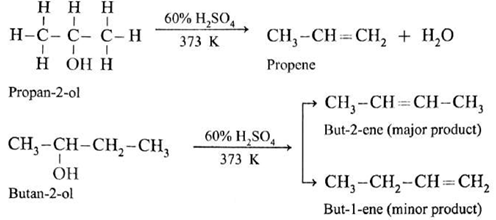
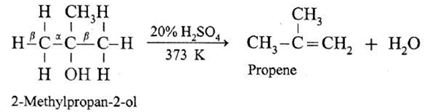
(c) A tertiary alcohol can be easily dehydrated by heating with 20% H2SO4 at 363K.
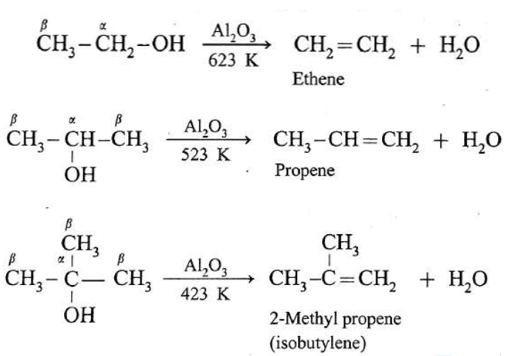
An alcohol can be dehydrated by passing vapours alcohols over heated alumina (AL2O3).
(iv) Oxidation of alcohols :
(a) Primary alcohol on oxidation with CrO3 forms aldehyde. However, a better reagent to bring about this oxidation is PCC (pyridinium chlorochromate).
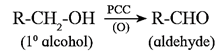
(b) Secondary alcohol on oxidation with chromic anhydride (CrO3) forms ketone.

(c) Tertiary alcohols are very difficult to oxidise. When tertiary alcohol is oxidised with powerful oxidising agents at high temperatures, C-C bonds are broken, yielding a variety of carboxylic acids with fewer carbon atoms than the original 3° alcohol.
Examples :
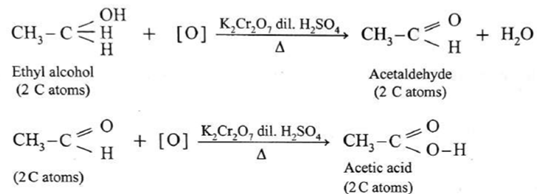
(a) Ethyl alcohol is a primary alcohol and on oxidation, it first forms acetaldehyde, which on further oxidation forms acetic acid. In this both, aldehyde and acid have same number of carbon atoms.
(b) Isopropyl alcohol is a secondary alcohol and on oxidation it gives a ketone, acetone with the same number of carbon atoms. Acetone resists further oxidation as it involves breaking of C—C bond.
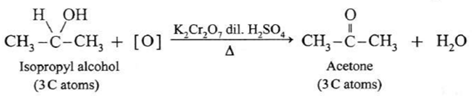
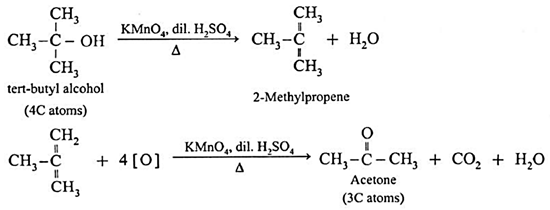
(c) The oxidation of tert-butyl alcohol is difficult, since it does not have α-hydrogen atom. It is oxidised by using acidic and stronger oxidising agents like KMnO4, CrO3 at high temperature, which dehydrate tertiary alcohol to alkene and then oxidise it to a ketone, acetone with less number of carbon atoms.
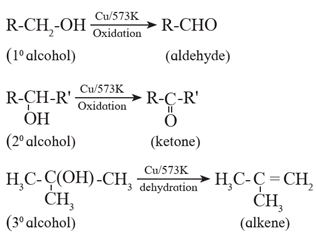
Heating with Cu : When vapours of primary, secondary and tertiary alcohols are passed over heated copper at 573 K, dehydrogenation (oxidation) of primary and secondary alcohol takes place while tertiary alcohols undergo dehydrogenation to give an alkene.
Reactions of phenols :
Phenol undergoes electrophilic substitution reactions more readily as compared to benzene. The -OH group in phenol is ring activating and an ortho-/paradirecting group.
(i) Halogentaion of phenol:
When phenol is treated with bromine water, a yellowish white precipitate of 2,4,6-tribromophenol is formed.
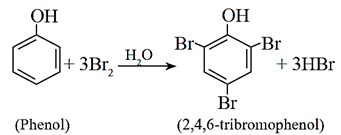
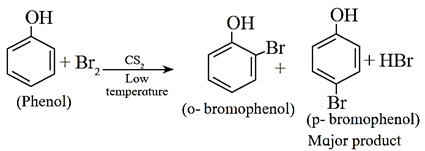
If the reaction is carried out in a solvent of lower polarity than water, such as CHCl3, CCl4 or CS2, a mixture of ortho- and parabromophenol is formed.
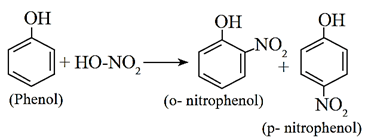
(ii) Nitration of phenol :
When phenol is treated with dilute nitric acid, a mixture of o-nitrophenol and p- nitrophenol is formed, In this reaction, p-nitrophenol is formed as the major product.
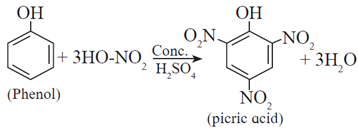
When phenol is warmed with a mixture of conc. nitric acid and conc. sulphuric acid (a nitrating mixture or the mixed acid), 2,4,6-trinitrophenol, commonly called picric acid, is formed.
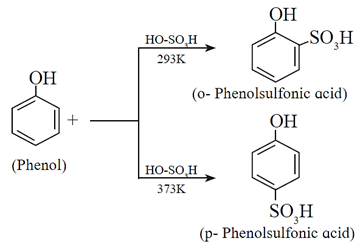
(iii) Sulfonation of phenol : Phenol reacts with concerntrated sulfuric acid at room temperature to give o-phenolsulfonic acid and at 373K, p-phenol sulfonic acid.
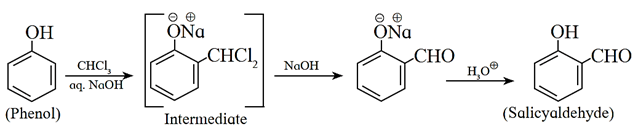
(iv) Reimer-Tiemann Reaction : When phenol is treated with chloroform in aqueous sodium hydroxide solution followed by hydrolysis with acid, salicylaldehyde (2-hydroxy benzaldehyde) is formed. This reaction is known as Reimer-Tiemann reaction.
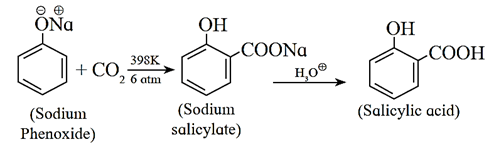
(v) Kolbe reaction : When phenol reacts with sodium hydroxide, sodium phenoxide is obtained. Phenoxide ion being more reactive than phenol towards electrophilic substitution, Phenoxide undergoes electrophilic substitution with carbon dioxide at 398 K under pressure of 6 atm (a weak electrophile) followed by acid hydrolysis, salicylic acid is formed as major product.
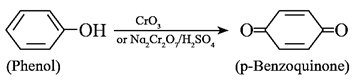
(vi) Oxidation of phenol : Phenol on oxidation with chromic anhydride or sodium dichromate in presence of H2SO4 gives p-benzoquinone. Phenol oxidizes slowly giving a dark coloured mixture in presence of air.
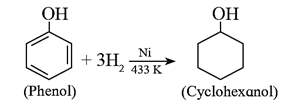
(vii) Catalytic hydrogenation of phenol: Phenol on catalytic hydrogenation gives cyclohexanol. In this reaction a mixture of vapours of phenol and hydrogen is passed over nickel catalyst at 433 K.
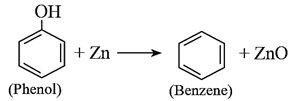
(viii) Reduction of phenol : Phenol is reduced to benezene on heating with zinc dust.
Ethers :
Preparation of ethers :
(i) By Dehydration of alcohols : When excess of ethyl alcohol is distilled with dehydrating agent like concentrated sulphuric acid (H2SO4) at 413 K, diethyl ether ethoxyethane is formed.
![]()
Mechanism of dehydration of alcohol to give ether :
Dehydration of alcohols to form ether is SN? reaction. The mechanism of dehydration of ethanol involves the following steps.
Step 1 (Protonation) : Initially ethyl alcohol gets protonated in the presence of acid to form ethyl oxonium ion.
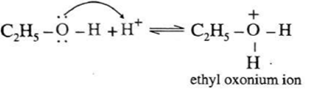
Step 2 (SN2 mechanism) : Protonated alcohol species undergoes a backside attack by second molecule of alcohol is a slow step.
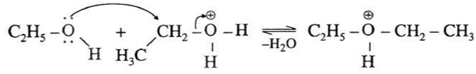
Step 3 (Deprotonation) : Formation of diethyl ether by elimination of proton
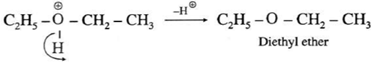
(i) Williamson’s synthesis : When an alkyl halide(R—X) is heated with sodium alkoxide(R—O-Na), an ether is obtained, this reaction is known as Williamson’s synthesis.
- This method is used to prepare simple(or symmetrical) ethers and mixed (unsymmetrical) ethers.
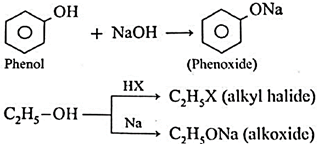
Sodium alkoxide is obtained by a reaction of sodium with an alcohol.
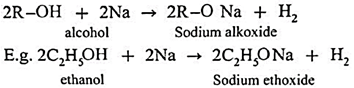
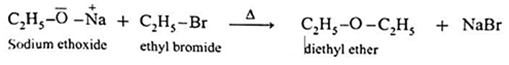
(a) Simple(Symmetrical) ether : When an alkyl halide and sodium alkoxide having similar alkyl groups are heated, symmetrical ether is obtained.
![]()
Sodium ethoxide on heating with ethyl bromide gives diethyl ether.
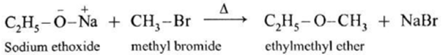
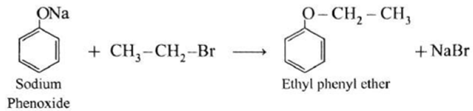
(b) Mixed(Unsymmetrical) ether : When an alkyl halide and sodium alkoxide or sodium phenoxide having different alkyl groups are heated, unsymmetrical ether (dialkyl ethers or alkyl aryl ether) is obtained.

Sodium ethoxide on heating with methyl bromide gives ethyl methyl ether.
Sodium phenoxide on heating with ethyl bromide gives ethyl phenyl ether.
Limitations of Williamson’s synthesis :
- In the preparation of unsymmetrical ethers by Williamson’s synthesis, the proper choice of the reactants namely alkyl halide and sodium alkoxide is necessary.
- The best yield of unsymmetrical ether is obtained when primary alkyl halide and tertiary alkoxide are heated, since primary alkyl halides are more susceptible to SN2
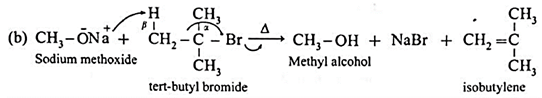
- Secondary or tertiary alkyl halide undergo α and β(halogen and hydrogen) elimination reaction giving an alkene instead of an ether since α carbon atom is sterically hindered by bulky alkyl groups.
t-butyl methyl ether can be synthesised by reaction of methyl bromide with sodium t-butoxide.
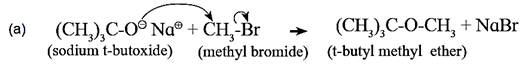
When tert.butyl bromide is treated with sodium methoxide, isobutylene is obtained.
Question : Explain Aryl halides do not give Williamson’s synthesis.
Ans. Williamson synthesis is effectively a method of preparation of ethers from two hydroxy compounds. The two substrates of Williamson synthesis, namely the nucleophile and alkyl halides are obtained from hydroxyl compounds as shown below:
Physical properties :
(i) Physical states and boiling points :
- Dimethyl ether and ethyl methyl ether are gases. Other ethers are colourless liquids with pleasant odour.
- Lower ethers are highly volatile and highly inflammable substances.
- Boiling points of ethers show gradual increase with the increase in molecular mass.
| Ether | B.P. / K |
| CH3-O-CH3 | 248 |
| C2H5-O-CH3 | 284 |
| C2H5-O- C2H5 | 308 |
- The solubility/miscibility of ethers in water is similar to that of alcohols of comparable molecular mass.
(ii) Polarity and solubility : Since bond angle is 110° and not 180°, the bond dipole moments of the two C-O bonds do not cancel each other; therefore ethers posses a small net dipole moment.
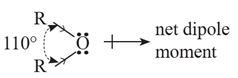
The solubility/miscibility of ethers in water is similar to that of alcohols of comparable molecular mass. This is because ethers can form hydrogen bonds with water through ethereal oxygen.
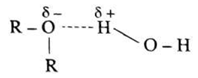
For example, diethyl ether and n-butyl alcohol have respective miscibilities of 7.5 and 9 g per 100 g of water.
Chemical properties of ethers :
(i) Laboratory test for ethers :
- Ethers are neutral compounds in aqueous medium.
- Ethers do not react with bases, cold dilute acids, reducing agents, oxidizing agents and active metals.
- However, ethers dissolve in cold concentrated H2SO4 due to formation of oxonium salts.
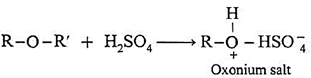
- This property distinguishes ethers from hydrocarbons.
(ii) Reaction involving alkyl group of ether :
Formation of peroxide : Ethers combine with atmospheric oxygen to form peroxide. Peroxides are hazardous because they decompose violently at high temperature.
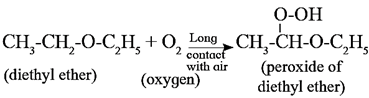
(iii) Reaction involving C-O bond
Reaction with hot dilute sulphuric acid (Hydrolysis) : Ethers when heated with dilute sulfuric acid undergo hydrolysis to give alcohols/phenols.
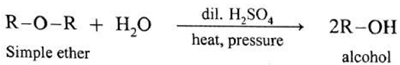
Examples :
(a) Dimethyl ether on hydrolysis give methanol.

(b) Diethyl ether on hydrolysis give ethanol.
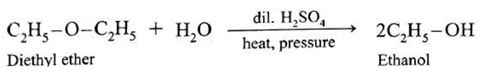
(c) A mixed ether on heating with dil. H2SO4 under pressure undergoes hydrolysis to give mixture of two different alcohols.
![]()
Ethyl methyl ether on hydrolysis give a mixture of ethanol and methanol.

(d) Anisole on hydrolysis give a mixture of phenol and methanol.
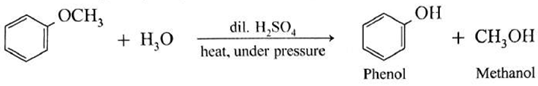
Reaction with PCl5 : Ethers react with PCl5 to give alkyl chlorides
R-O-R' + PCl5 \(\underrightarrow{Δ}\) R-Cl + R'-Cl + POCl3
Examples :
(a) Action of phosphorus pentachloride on Diethyl ether : When diethyl ether is heated with ethyl methyl ether, ethyl chloride is formed.
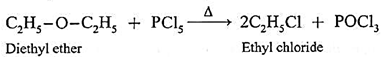
(b) Action of phosphorus pentachloride on Ethyl methyl ether : When ethyl methyl ether is heated with phosphorus pentachloride, a mixture of ethyl chloride and methyl chloride is formed.
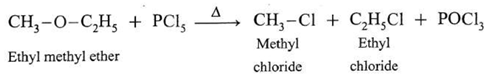
(c) Action of phosphorus pentachloride on ethyl phenyl ether (Anisole) : When methyl phenyl ether is heated with phosphorus pentachloride, a mixture of methyl chloride, chlorobenzene is formed.
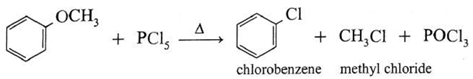
(iii) Reaction with hot concentrated acid :
Alkyl ethers react with hot and concentrated HI and HBr to give an alcohol and an alkyl halide.
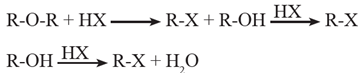
The order of reactivity of HX is HI>HBr>HCl
Aryl alkyl ethers have stronger and shorter bond between oxygen and the aromatic ring. Hence an aryl alkyl ether undergoes cleavage of oxygen - alkyl bond and yields a phenol and an alkyl halide on reaction with HI.
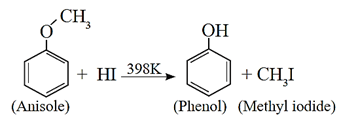
Examples :
(a) Action of hydroiodic acid on Diethyl ether : When diethyl ether (ethoxy ethane) is treated with hydroiodic acid, a mixture of ethanol and ethyl iodide is formed.
C2H5—O—C2H5 + HI → C2H5—OH + C2H5—I
If excess of hydroiodic acid is available, then ethyl alcohol further reacts with hydroiodic acid at higher temperature to form ethyl iodide and water.
C2H5—OH + HI → C2H5—I + H2O
(b) Action of hydroiodic acid on Ethyl methyl ether : When ethyl methyl (methoxy ethane) is treated with hydroiodic acid, a mixture of ethyl alcohol and methyl iodide is formed.
C2H5—O—CH3 + HI → C2H5—OH + CH3I
If excess of hydroiodic acid is available, then ethyl alcohol further reacts with hydroiodic acid at higher temperature to form ethyl iodide and water.
C2H5—OH + HI → C2H5I + H2O
(c) Action of hydroiodic acid on Methyl phenyl ether (anisole) : When methyl phenyl ether (anisole) is treated with hydroiodic acid, phenol and methyl iodide is formed. Here, phenol does not react further with HI because —OH group is attached to sp2-hybridised carbon atom and it cannot be replaced by iodide (nucleophile).
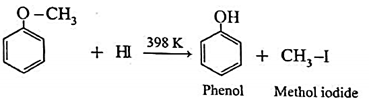
(iv) Electrophilic substitution in aromatic ethers : The alkoxy group in aromatic
ether is a ring activating and ortho-, paradirecting group toward electrophilic aromatic substitution.
Resonance structures:
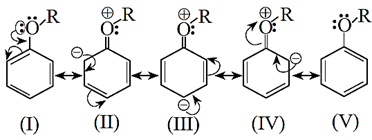
+R Effect of -OR group results in increased electron density at the para- and two orthopositions (see resonance structures II, III and IV).
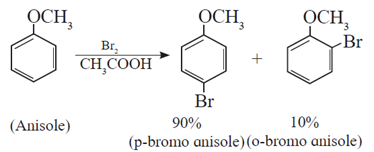
(a) Halogenation : Anisole undergoes bromination with bromine in acetic acid even in the absence of ferric bromide catalyst. It is due to activation of benzene ring by the methoxy group.
When anisole is treated with bromine in acetic acid, p-bromoanisole (major product) is obtained.
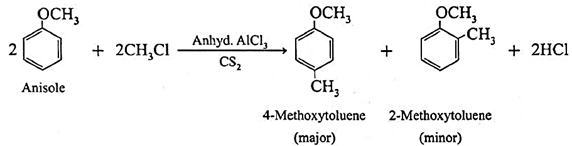
(b) Friedel Crafts reaction : When anisole is treated with alkyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as catalyst, 4-Methoxy toluene is formed as major product. The alkyl groups are introduced at -ortho and -para positions in anisole, the reaction is known as Friedel-Crafts alkylation reaction.
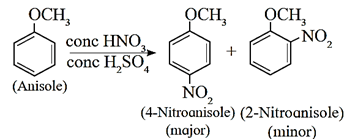
(c) Nitration : Anisole reacts with concentrated nitric acid in presence of concentrated sulfuric acid (Nitrating mixture) to give a mixture of o-nitro anisole and p-nitro anisole.
Uses of alcohols, phenols and ethers :
Methyl alcohol :
- Methyl alcohol is used in industry to dissolve oils, fats, gums, and other substances.
- It is utilised in dry cleaning as well as perfume and varnish preparation.
- At low temperatures, it is utilised as an antifreeze agent in vehicle radiators.
- It is used to make methyl chloride, dimethyl sulphate, and formaldehyde.
- It is used in the denaturement of ethyl alcohol.
Ethyl alcohol :
- Ethyl alcohol is used as solvent for dyes, oils, perfumes, cosmetics and drugs.
- A mixture of 10-20% ethyl alcohol with petrol is used as motor fuel.
- A mixture of ethyl alcohol and calcium acetate in gel form is used as solid fuel.
- It is widely used in beverages.
- Since ethyl alcohol has low freezing point, it is used in thermometer.
- It is an effective tropical antiseptic therefore it is used in many mouth washes.
- It kills micro-organisms on wound surface and in the mouth but its low toxicity does not kill the cells of the skin or mouth tissues.
- It is used in the preparation of chloroform, iodofonn, acetic acid and ethers.
- It is used as fuel.
Phenol :
- Phenol is used in preparation of phenol formaldehyde resin For example : bakelite.
- Phenols are used as antiseptic in common products like air fresheners, deodorants, mouthwash, calamine lotions, floor cleaners, etc.
- It is used in the preparation of phenolphthalein—an indicator and in certain dyes.
- It is used in the preparation of drugs such as salol, aspirin, etc.
- It is used in the preparation of 2,4-dichlorophenoxy acetic acid which is used as selective weed killer
- It is used to prepare picric acid which is used as explosive.
Ethers :
- Earlier diethyl ether was used as a general anaesthetic in surgical operations.
- Diethyl ether is used as a solvent for Grignard reagents, fats, waxes, oil, etc.
- It is used as refrigerant.
- Natalite, a mixture of diethyl ether and ethyl alcohol is used as fuel.
PDF : Chapter-11-Alcohols, Phenols and Ethers-Text Book
PDF : Chapter-11-Alcohols, Phenols and Ethers- Notes
PDF : Chapter-11-Alcohols, Phenols and Ethers- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 10- Halogen Derivatives – Online Notes
Next Chapter : Chapter-12-Aldehydes, Ketones and Carboxylic acids- Online Notes
We reply to valid query.