Transition and Inner transition Elements
Maharashtra Board-Class-12-Chemistry-Chapter-8
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
- The transition elements belong to d block of the periodic table.
- Transition metal atom has an incomplete d-subshell or it give cations with incomplete d subshell.
d-block elements : d-block elements are defined as the elements in which the differentiating electron enters d-orbital of the penultimate shell i.e. (n−1) d-orbital where ‘n’ is the last shell.
The general electronic configuration can be represented as, (n−1)d1−10, ns1−2 .
They are all metal
Position in the periodic table
- d-block elements exhibit properties between those of s and p block elements.
- Hence, they show a transition in the properties from those of the most electropositive s-block elements and less electropositive (or electronegative)p-block elements.
- Therefore, most of the d-block elements are called transition elements.
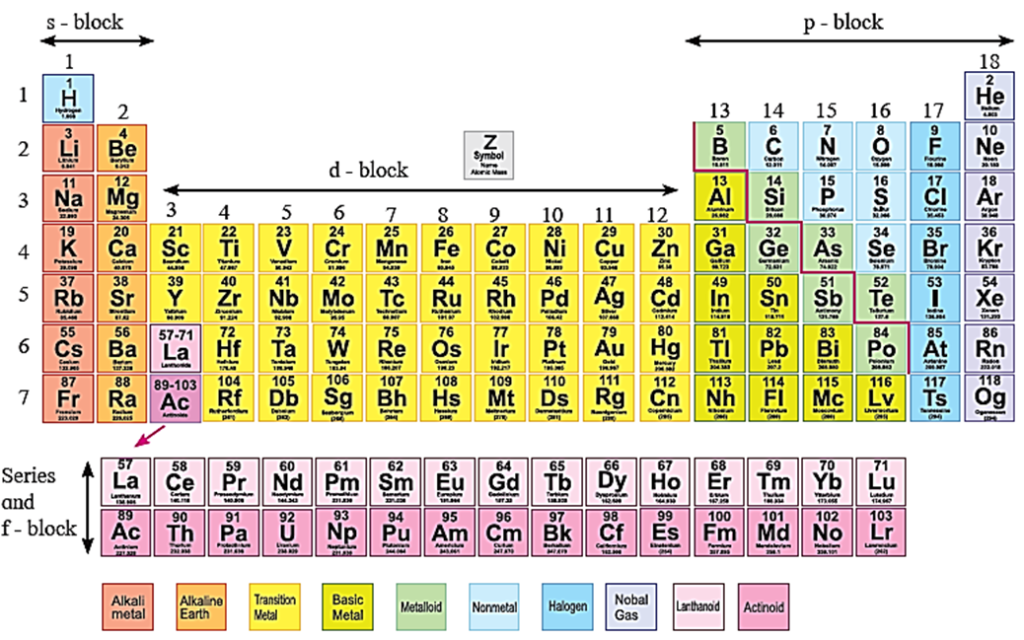
- The transition elements of the modern periodic table appear as groups 3 to 12 or as four long periods 4 to 7.
Position of d-block elements in the modern periodic table :
Electronic configuration :
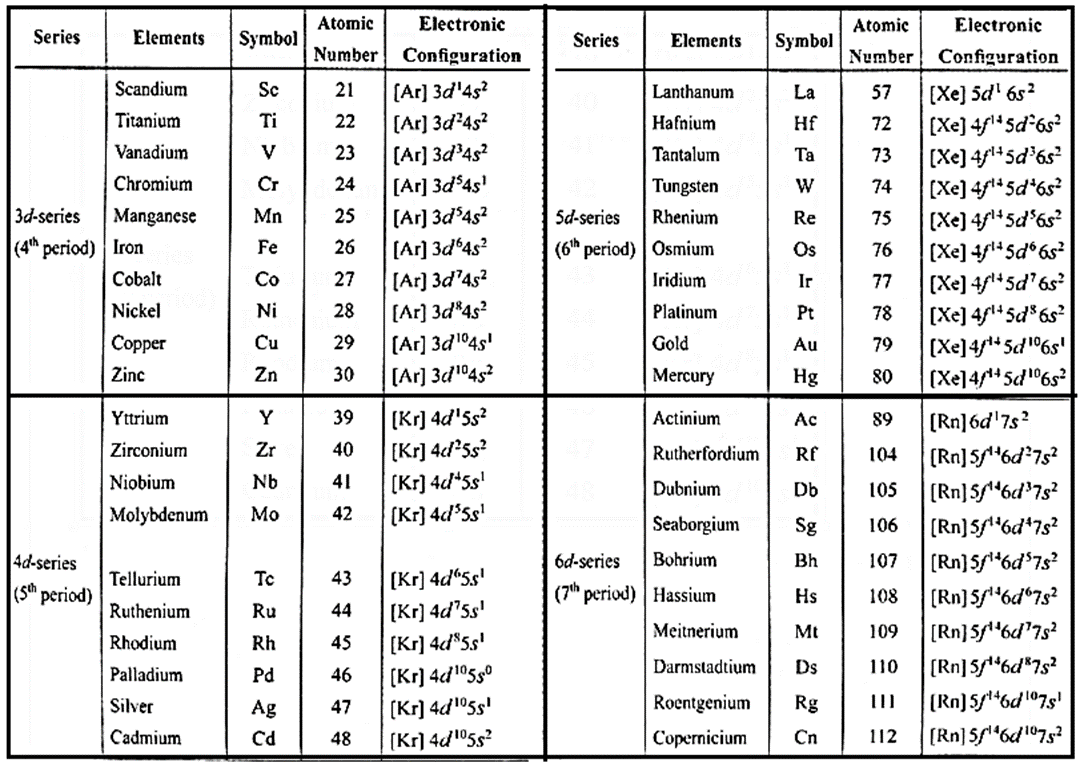
General electronic configuration of four series of d-block elements of periodic table can be represented as given below:
| d-series | Periods | Electronic configuration |
| 3d series | 4th | [Ar] 3d1-10 4s1-2 |
| 4d series | 5th | [Kr] 4d1-10 5s1-2 |
| 5d series | 6th | [Xe] 4f14 5d1-10 6s1-2 |
| 6d series | 7th | [Rn] 5f14 6d1-10 7s2 |
Condensed Electronic configuration of each series of d-block elements :
- An element whose differentiating electron is a 4d-electron will be present in fifth period of the periodic table.
Transition elements with electronic configuration 3d44s2 and 3d94s2 do not exist :
Reason :
- d-orbitals are degenerate orbitals and they acquire extra stability when half filled (3d5) or completely filled (3d10). Hence 3d4 and 3d9 electronic configurations are less stable.
- The energy difference between 3d and 4s subshells is very low, hence there arises a transfer of one electron from 4s orbital to 3d orbital.
The electronic configuration changes as,
3d44s2 → 3d54s1
3d94s2 → 3d104s1
Therefore transition elements, with electronic configurations 3d44s2 and 3d94s2 do not exist.
Electronic configuration of 3d series of d-block elements :
| Elements | Symbol | At. No. | Expected electronic configuration | Observed electronic configuration |
| Scandium | Sc | 21 | [Ar] 3d1 4s2 | [Ar] 3d1 4s2 |
| Titanium | Ti | 22 | [Ar] 3d2 4s2 | [Ar] 3d2 4s2 |
| Vanadium | V | 23 | [Ar] 3d3 4s2 | [Ar] 3d3 4s2 |
| Chromiun | Cr | 24 | [Ar] 3d4 4s2 | [Ar] 3d5 4s1 |
| Manganese | Mn | 25 | [Ar] 3d5 4s2 | [Ar] 3d5 4s2 |
| Iron | Fe | 26 | [Ar] 3d6 4s2 | [Ar] 3d6 4s2 |
| Cobalt | Co | 27 | [Ar] 3d7 4s2 | [Ar] 3d7 4s2 |
| Nickel | Ni | 28 | [Ar] 3d8 4s2 | [Ar] 3d8 4s2 |
| Copper | Cu | 29 | [Ar] 3d9 4s2 | [Ar] 3d10 4s1 |
| Zinc | Zn | 30 | [Ar] 3d10 4s2 | [Ar] 3d10 4s2 |
Remember : Any subshell having a half filled or completely filled electronic configuration has extra stability.
Electronic configuration of chromium and copper :
(i) Chromium (24Cr) : Chromium has electronic configuration,
Expected : 1s22s22p63s23p63d44s2
Observed : 1s22s22p63s23p63d54s1
Explanation :
- The energy difference between 3d- and 4s-orbitals is very low.
- d-orbitals being degenerate, they acquire more stability when they are half-filled (3d6).
- Therefore, there arises a transfer of one electron from 4s-orbital to 3d-orbital in Cr giving more stable half-filled orbital. Hence, the configuration of Cr is [Ar] 3d54s1 and not [Ar] 3d44s2.
(ii) Copper (29Cu) : Copper has electronic configuration,
Expected : 1s22s22p63s23p63d94s2
Observed : 1s22s22p63s23p63d104s1
Explanation :
- The energy difference between 3d- and 4s-orbitals is very low.
- d-orbitals being degenerate they acquire more stability when they are completely filled.
- Therefore, there arises a transfer of one electron from 4s-orbital to 3d-orbital in Cu giving completely filled more stable d-orbital.
- Hence the configuration of Cu is [Ar] d104s1 and not [Ar] 3d94s2
Oxidation states of first transition series :
- One of the notable features of transition elements is the great variety of oxidation states they show in their compounds.
- The transition metals (or elements) exhibit variable oxidation states due to their electronic configuration, (n − 1)d1−10ns1−2. i.e. 3d1−104s1−2 for the first row.
- They show only positive oxidation states due to loss of electrons from outer 4s-orbital and the penultimate 3d-orbital.
- Loss of one 4s electron forms M+ ion. Loss of two 4s electrons form M2+ ion.
- +2 is the common oxidation state ottliese elements.
- Higher oxidation states are due to loss of 3d-electrons along with 4s electrons.
- As the number of unpaired electrons increases, the number of oxidation states shown by the element also increases
- Sc has only one unpaired electron and it shows two oxidation states (+2 and +3)
- Mn with 5 unpaired d electrons show six different oxidation states. They are +2, +3, +4, +5, +6 and +7. Thus Mn has the highest oxidation state.
- From Fe onwards variable oxidation states decreases as the number of unpaired electron decreases.
- The last element in the series, Zn shows only one oxidation state (+2).
Table : Oxidation states of first transition series elements
| Elements | Outer elecrtonic configuration | Oxidation states |
| Sc | 3d1 4s2 | +2, +3 |
| Ti | 3d2 4s2 | +2, +3, +4 |
| V | 3d3 4s2 | +2, +3, +4, +5 |
| Cr | 3d5 4s1 | +2, +3, +4, +5, +6 |
| Mn | 3d5 4s2 | +2, +3, +4, +5, +6, +7 |
| Fe | 3d6 4s2 | +2, +3, +4, +5, +6 |
| Co | 3d7 4s2 | +2, +3, +4, +5 |
| Ni | 3d10 4s2 | +2, +3, +4 |
| Cu | 3d10 4s1 | +1, +2 |
| Zn | 3d10 4s2 | +2 |
Electronic configuration of various ions of 3d elements :
| Elements | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | |
| Atomic no : | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | |
| Species | Valence shell Electronic Configuration | ||||||||||
| M | 3d1 4s2 | 3d2 4s2 | 3d3 4s2 | 3d5 4s2 | 3d5 4s2 | 3d6 4s2 | 3d1 4s2 | 3d8 4s2 | 3d10 4s1 | 3d10 4s2 | |
| M+ | 3d1 4s1 | 3d2 4s1 | 3d3 4s1 | 3d5 | 3d5 4s1 | 3d6 4s1 | 3d7 4s1 | 3d8 4s1 | 3d10 4s0 | 3d10 4s1 | |
| M2+ | 3d2 | 3d2 | 3d3 | 3d4 | 3d5 | 3d6 | 3d7 | 3d8 | 3d9 | 3d10 | |
| M3+ | [Ar] | 3d1 | 3d2 | 3d3 | 3d4 | 3d5 | 3d6 | 3d7 | - | - | |
Some features of Oxidation state of various ions of 3d elements :
Oxidation state of Scandium :
- Scandium has electronic configuration, 21Sc : 1s22s22p63s23p63d14s2
- Sc shows only two oxidation states namely +2 and +3.
- Due to the loss of two electrons from the 4s-orbital, Sc acquires +2 oxidation state Sc2+ : 1s22s22p63s23p63d1
- Due to the loss of one more electron from the 3d-orbital, it acquires +3 oxidation state with the extra stability of an inert element 18Ar. Sc3+ : 1s22s22p63s23p6.
- Since Sc3+ acquires extra stability of inert element [Ar]18, it does not form higher oxidation state.
Oxidation state of chromium :
- The observed electronic configuration of chromium is, 24Cr [Ar] 3d54s1
- Different possible oxidation states of Cr are +1 (3d5), +2 (3d4), +3 (3d3), +4 (3d2), +5 (3d1) and +6 (3d0).
- Although in +1 state, Cr gets extra stability of half filled 3d5-orbital, it does not exhibit +1l state in common except with pyridine.
- Cr2+ has few stable salts like CrCl2, CrSO4 while Cr3+ forms very stable salts like CrCl3.
- Cr4+ and Cr5+ are unstable oxidation states.
- Cr6+ is the most stable state clue to inert gas [Ar] electronic configuration and forms the salts like K2Cr2O7
Oxidation state of manganese :
- The electronic configuration of Mn is 25Mn [Ar] 3d54s2
- Mn shows oxidation states ranging from +2 to +7.
- Mn has stable half-filled d-subshell.
- In +2 and +3 oxidation states, the electronic configuration of Mn is, Mn2+ [Ar] 3d5 and Mn3+ [Ar] 3d4
- Since half-filled d-orbital (3d5) has more stability and lower energy than 3d4, Mn2+ is more stable than Mn3+
- Due to a small difference in energy between 3d and 4s-orbitals, Mn can lose or share electrons from both the orbitals, hence shows variable oxidation states.
Oxidation state of iron :
- The electronic configuration of Fe is 26Fe [Ar] 3d64s2
- Oxidation states of iron are +2, +3, +4, +5, +6
- 26Fe : 1s22s22p63s23p63d64s2
- Fe2+ : 1s22s22p63s23p63d6
- Fe3+ : 1s22s22p63s23p63d5
- In +2 and +3 oxidation states of Fe, the electronic configuration is, Fe2+ [Ar] 3d6 and Fe3+ [Ar] 3d5
- Since half-filled d-orbital is more stable, +3 state of Fe is more stable than +2 state.
Oxidation state of zinc :
- The electronic configuration of zinc is, 30Zn 1s22s22p63s23p63d104s2 or [Ar] 3d104s2
- Due to loss of two electrons from 4s subshell Zn shows oxidation state +2, with electronic configuration, [Ar]183d10.
- Since Zn+2 acquires an extra stability of completely filled 3d10 -orbital, it shows only one oxidation state +2.
Physical properties of first transition series
- All transition elements of the first series are metals.
- Except Zn, they are very hard and have low volatility.
- They show characteristic properties of metals. They are lustrous, malleable and ductile.
- They are good conductors of heat and electricity.
- They have high melting points and boiling points.
- Except Zn and Mn, they have one or more typical metallic structures at normal temperatures.
- In 3d transition elements, (i) Scandium (Sc) has lowest density and (ii) Zinc (Zn) has the highest density.
- The densities of a’-block elements are higher than s-block elements due to higher nuclear charge which results in reduction in atomic size.
Trends in melting points of transition elements :
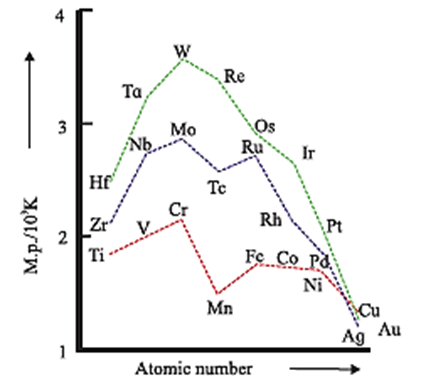
- All transition elements are metals and the strength of metallic bonding increases as the number of unpaired electrons increase.
- In transition elements as atomic number increases, the number of unpaired electrons increases from (n − 1) d1 to (n − 1) d5.
- For example in 3d-series, melting points increase from 21Sc to 24Cr in 4d-series from 39Y to 42Mo, and in 5d-series from 72Hf to 74W.
- After (n − 1) d5 electronic configuration, the electrons start pairing, hence the number of unpaired electrons decreases, hence metallic character, melting points decrease from (n − 1) d6 to (n − 1) d10.
- In all transition series the melting point increases steadily up to d 5 configuration and after this melting point decreases regularly.
Trends in atomic properties of the first transition series :
Atomic and ionic radii
Trends in atomic radii of d-block elements :
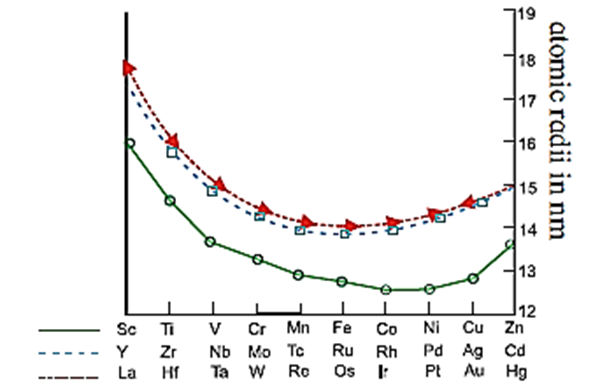
- The atomic radii of elements of transition elements are intermediate between those of s-block and p-block elements of the same period.
- Atomic radii of transition elements decrease with increase in atomic number.
- In transition elements the last electron enters (n − 1) d subshell. With the d-electrons after poor screening effective nuclear charge increases.
- Hence atomic radii decrease with increase in atomic number.
- The decrease in atomic radii is less. There is slight increase in atomic radii of last two elements Cu and Zn. u
Variation in atomic or ionic radii of 3d-series elements :
Ionic radii of transition elements show the same trend as of the atomic radii.
The elements of first transition series show variable oxidation states. The trends in ionic radii, thus, can be studied with (i) elements having same oxidation state or (ii) considering various oxidation states of the same element.
- The atomic or ionic radii of 3-d series transition elements are smaller than those of representative elements, with the same oxidation states.
- For the same oxidation state, there is an increase in nuclear charge and a gradual decrease in ionic radii of 3d-series elements is observed.
- Thus ionic radii of ions with oxidation state + 2 decreases with increase in atomic number.
- There is slight increase is observed in Zn2+ ions. With the higher oxidation states, effective nuclear charge increases.
- Therefore ionic radii decrease with increase in oxidation state of the same element. For example, Fe2+ ion has ionic radius 77 pm whereas Fe3+ has 65 pm.
Atomic properties of first transition series elements
| Element (M) | Atomic number (Z) | Density (g/cm3) | Atomic/ionic radius (pm) | Ionisation enthalpy (kJ/mol) | ||
| M | M2+ | M3+ | ||||
| Sc | 21 | 3.43 | 164 | - | 73 | 631 |
| Ti | 22 | 4.1 | 147 | - | 67 | 656 |
| V | 23 | 6.07 | 135 | 79 | 64 | 650 |
| Cr | 24 | 7.19 | 129 | 82 | 62 | 653 |
| Mn | 25 | 7.21 | 127 | 82 | 65 | 717 |
| Fe | 26 | 7.8 | 126 | 77 | 65 | 762 |
| Co | 27 | 8.7 | 125 | 74 | 61 | 758 |
| Ni | 28 | 8.9 | 125 | 70 | 60 | 736 |
| Cu | 29 | 8.9 | 128 | 73 | - | 745 |
| Zn | 30 | 7.1 | 137 | 75 | - | 906 |
Ionisation Enthalpy :
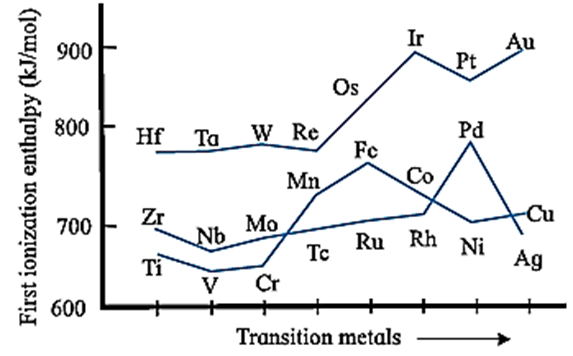
- The ionisation enthalpies of transition elements are quite high and lie between those of s-block and p-block elements.
- This is because the nuclear charge and atomic radii of transition elements lie between those of s-block and p-block elements.
- As atomic number of transition elements increases along the period and along the group, first ionisation enthalpy increases even though the increase is not regular.
- If IE1, IE2 and IE3 are the first, second and third ionisation enthalpies of the transition elements, then IE1 < IE2 < IE3.
- In the transition elements, the added last differentiating electron enters into (n − 1) d-orbital and shields the valence electrons from the nuclear attraction. This gives rise to the screening effect of (n − 1) d electrons.
- Due to this screening effect of (n − 1) d electrons, the ionisation enthalpy increases slowly and the increase is not very regular.
Why, the ionisation enthalpies of the elements of the third transition series are much higher than the first and second series ?
Reason :
- The atoms of elements of third transition series possess filled 4f- orbitals.
- 4f orbitals show poor shielding effect on account of their peculiar diffused shape.
- As a result, the valence electrons experience greater nuclear attraction. A greater amount of energy is required to ionize elements of the third transition series.
- The ionisation enthalpies of the elements of the third transition series are, therefore much higher than the first and second series.
Table : Ionisation enthalpies of first transition series elements
| Element →
IE ↓ |
Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn |
| IE1 | 632 | 659 | 650 | 652 | 717 | 762 | 756 | 736 | 744 | 906 |
| IE2 | 1245 | 1320 | 1376 | 1635 | 1513 | 1563 | 1647 | 1756 | 1961 | 1736 |
| IE3 | 2450 | 2721 | 2873 | 2994 | 3258 | 2963 | 3237 | 3400 | 3560 | 3838 |
(IE = Ionisation Enthalpy in kJ/mol)
| Know This :
The inner shell electrons in an atom screen or shield the outermost electron from nuclear attraction. This effect is called shielding effect. The magnitude of shielding effect depends upon the number of inner electrons. |
Metallic character :
Metallic character of transition metals :
- All the transition elements are metals.
- They are hard, lustrous, malleable, ductile and they have high tensile strength.
- They have high melting points and boiling points.
- Their metallic character is due to vacant or partially filled (n — 1) d—orbitals, and they involve both metallic and covalent bonding.
- Since the strength of metallic bonds depends upon the number of unpaired electrons, it increases up to middle i.e., up to (n − 1) d5, hence accordingly melting points and boiling points also increase.
- After (n − 1) d5 configuration, the electrons start pairing, hence the metallic strength, melting points and boiling points decrease with the increase in atomic number.
- Nearly all transition metals have simple hexagonal closed packed (hcp), cubic closed packed (ccp) or body centered cubic (bcc) lattices which are characteristic of true metals.
Remember : Hardness, high melting points and metallic properties of the transition elements indicate that the metal atoms are held strongly by metallic bonds with covalent character.
Variation in metallic character of 3d transition series elements :
- In 3d-series elements as atomic number increases from scandium (Sc [Ar]18 3d14s2) the number of unpaired electrons increases up to 3d5 in chromium.
- As the number of unpaired electrons increases, the metallic character increases, hence the melting points and boiling points increase from 21Sc (3d1) to 24Cr (3d5).
- After chromium the number of unpaired electrons goes on decreasing due to the pairing of electrons, hence metallic character, melting points and boiling points decrease from 25Mn to 29Cu.
- Zinc has all electrons paired, hence it is soft, has low melting and boiling points.
Magnetic Properties :
The compounds of transition metals exhibit magnetic properties due to the unpaired electrons present in their atoms or ions.
- Paramagnetic substances ; When a magnetic field is applied, substances which are attracted towards the applied magnetic field are called paramagnetic substances. Example : Ni2+, Pr4+
- Diamagnetic Substances : When a magnetic field is applied, substances which are repelled by the magnetic fields are called diamagnetic substances. Example : Zn2+, La3+
- Ferromagnetic substances : When a magnetic field is applied, substances which are attracted very strongly are called ferromagnetic substances. These substances can be magnetised. For example, Fe, Co, Ni are ferromagnetic.
Causes of Paramagnetism, ferromagnetism and diamagnetic
- Paramagnetism and ferromagnetism is due to the presence of unpaired electrons in species
- When there is no unpaired electron, i.e. all electron spins are paired, the species become diamagnetic
Transition metals Fe, Co, Ni :
- Among transition metals Fe, Co, Ni are ferromagnetic.
- When magnetic field is applied externally all the unpaired electrons in these metals and their compounds align in the direction of the applied magnetic field. Due to this the magnetic susceptibility is enhanced.
- These metals can be magnetized, that is, they acquire permanent magnetic moment.
Thorium (Rn) :
- The 90th element thorium has electronic configuration, [Rn] 6d27s2. Since it has 2 unpaired electrons it is paramagnetic.
Zinc metal and Zn++ ions :
- The electronic configurations of Zn atom and Zn++ ion are
30Zn [Ar] 3d104s2
Zn++ [Ar] 3d10
- Since all electrons in zinc metal and Zn++ ions are paired, they are diamagnetic.
Vanadium :
- The electronic configuration of vanadium is, 23V [Ar] 3d34s2.
- In + 5 oxidation state, the electronic configuration is, V5+ [Ar].
- Since in V5+ state, vanadium has all electrons paired, it is diamagnetic
Magnetic properties of transition (or d-block) elements :
- Most of the transition metal ions and their compounds are paramagnetic in nature due to the presence of one or more unpaired electrons in their (n — 1) d-orbitals. Hence they are attracted in the magnetic field.
- As the number of unpaired electrons increases from 1 to 5 in d-orbitals, the paramagnetic character and magnetic moment increase.
- The transition elements or their ions having all electrons paired are diamagnetic and they are repelled in the magnetic field.
- Metals like Fe, Co and Ni possess very high paramagnetism and acquire permanent magnetic moment hence they are ferromagnetic.
Magnetic moment :
The magnetic moment is expressed in Bohr magneton (B.M.). It is denoted by μ
Bohr magneton (B.M.) is a unit of magnetic moment : 1 B.M. = \(\frac{eh}{4πm_ec}\)
where, h : Planck’s constant (h = 6.626 x 10−34 J s)
e : electronic charge (1.60218 x 10−19 C)
me : mass of an electron (9.109 x 10−31 kg)
c : velocity oflight. (2.998 x 108 m s−1)
A single unpaired electron has magnetic moment μ = 1.73 BM.
Effective magnetic moment of the species :
- The magnetic moment in the species arises due to the presence of unpaired electrons.
- The magnetic moment depends upon the sum of orbitals and spin contribution for each unpaired electron present in the species.
- In transition metal ions, the contribution of orbital magnetic moment is suppressed by the electrostatic field of other atoms, molecules or ions surrounding the metal ion in the compound.
- Hence the net or effective magnetic moment arises mainly due to spin of electrons. The effective magnetic moment μeff of a paramagnetic substance is given by ‘spin only’ formula represented as,
μ = \(\sqrt{n(n+2)}\) B.M., where n is the number of unpaired electrons.
Importance of magnetic moment (μ) :
- From the measurements of the magnetic moment (ii) of the species or metal complexes of the first row of transition elements, the number of unpaired electrons can be calculated with the spin-only formula.
- As magnetic moment is directly related to the number of unpaired electrons, value of it will vary directly with the number of unpaired electrons.
- In 2nd and 3rd transition series, orbital angular moment is significant. Hence spin-only formula for the complexes of 2nd and 3rd transition series is not useful.
Magnetic moments of ions of first transition series elements (values in BM):
| Ion | Outer electronic configuration | Number of unpaired electrons | Calculated value of magnetic moment | Experimental value |
| Sc3+ | 3d0 | 0 | 0 | 0 |
| Ti3+ | 3d1 | 1 | 1.73 | 1.75 |
| V3+ | 3d2 | 2 | 2.84 | 2.76 |
| Cr3+ | 3d3 | 3 | 3.87 | 3.86 |
| Cr2+ | 3d4 | 4 | 4.90 | 4.80 |
| Mn2+ | 3d5 | 5 | 5.92 | 5.96 |
| Fe2+ | 3d6 | 4 | 4.90 | 5.3-5.5 |
| Co2+ | 3d7 | 3 | 3.87 | 4.4-5.2 |
| Ni2+ | 3d8 | 2 | 2.84 | 2.9-3.0, 4.0 |
| Cu2+ | 3d9 | 1 | 1.73 | 1.8-2.2 |
| Zn2+ | 3d10 | 0 | 0 | 0 |
Magnetic moments are determined experimentally in solution or in solid state where the central atom or ion is hydrated or bound to ligands. Hence a slight difference is observed in calculated and experimentally obtained values of magnetic moment (μ).
Colour :
- A substance appears coloured if it absorbs a portion of visible light.
- The colour depends upon the wavelength of absorption in the visible region of electromagnetic radiation.
- The white colour of a compound indicates the absorption of uv radiation.
Compounds (or ions) of many d-block elements or transition metals are coloured :
Reason :
- This is due to the presence of one or more unpaired electrons in (n − 1) d-orbital. The transition metals have incompletely filled (n − 1) d-orbitals.
- The energy required to promote one or more electrons within the d-orbitals involving d-d transitions is very low.
- The energy changes for d-d transitions lie in visible region of electromagnetic radiation.
- Therefore transition metal ions absorb the radiation in the visible region and appear coloured.
- Colour of ions of d-block elements depends on the number of unpaired electrons in (n − 1) d-orbital. The ions having equal number of unpaired electrons have similar colour.
- The colour of metal ions is complementary to the colour of the radiation absorbed.
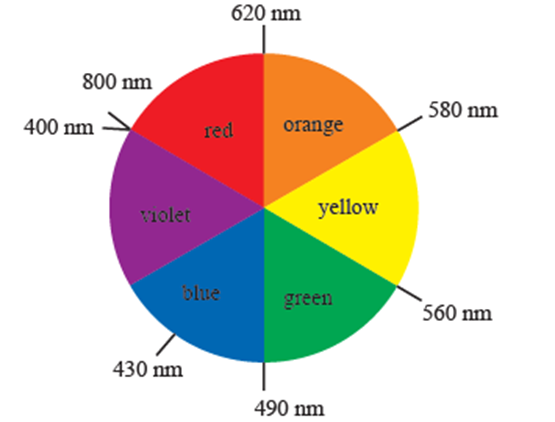
Identification of complementary colour of a compound :
- The transition metal ions absorb the radiation in the visible region and appeared coloured.
- Metal ion absorbs radiation of certain wavelength from the visible region. Remaining light is transmitted and the observed colour corresponds to the complementary colour of the light observed.
- The complementary colour can be identified (with the diagram given).
- For example if red colour is absorbed then transmitted complementary colour is green.
Colour of 3d transition metal ions :
| Ion | Outer electronic configuration | Number of unpaired electrons | Colour |
| Sc3+ | 3d0 | 0 | Colourless |
| Ti3+ | 3d1 | 1 | Purple |
| Ti4+ | 3d0 | 0 | Colourless |
| V3+ | 3d2 | 2 | Green |
| Cr3+ | 3d3 | 3 | violet |
| Mn2+ | 3d5 | 5 | Light pink |
| Mn3+ | 3d4 | 4 | Violet |
| Fe2+ | 3d6 | 4 | Pale green |
| Fe3+ | 3d5 | 5 | Yellow |
| Co2+ | 3d7 | 3 | Pink |
| Ni2+ | 3d8 | 2 | Green |
| Cu2+ | 3d9 | 1 | Blue |
| Cu+ | 3d10 | 0 | Colourless |
| Zn2+ | 3d10 | 0 | Colourless |
The factors on which the colour of transition metal ion depends :
- The presence of incompletely filled d-orbitals in metal ions. (The compounds with the configuration d0 and d10 are colourless.)
- The presence of unpaired electrons in d-orbitals.
- d → d transitions of electrons due to absorption of radiation in the visible region.
- Nature of groups (anions) (or ligands) linked to the metal ion in the compound or a complex.
- Type of hybridisation in metal ion in the complex.
- Geometry of the complex of the metal ion.
Example :
- When cobalt chloride (Co2+) is dissolved in water, it forms a pink solution of the complex [Co(H2O)6] 2+ which has octahedral geometry.
- But when this solution is treated with concentrated hydrochloric acid, it turns deep blue.
- This change is due to the formation of another complex [CoCl4]2− which has a tetrahedral structure.
[Co(H2O)6] 2+ + 4Cl− → [CoCl4] 2− + 6H2O
Know This :
|
Catalytic Properties : Transition metals and their compounds exhibit good catalytic properties. They have proven to be good homogeneous and heterogeneous catalysts.
Catalytic properties of the d-block or transition metals :
- d-block elements or transition metals and their compounds or complexes influence the rate of a chemical reaction and hence act as catalysts.
- In homogeneous catalysis a catalyst forms an unstable intermediate compound which decomposes into products and regenerates the catalyst. But transition metals involve heterogeneous catalysis.
- The transition metals have incompletely filled a’-subshells which adsorb reactantants on the surface and provide large surface area for the reactants to react.
- Since transition metals have variable oxidation states they are very good catalysts.
- Hence, compounds of Fe, Co, Ni, Pt, Pd, Cr etc are used as catalysts in many reactions.
Use of different transition metals as catalysts :
- MnO2 acts as a catalyst for decomposition of KClO3.
- In manufacture of ammonia by Haber’s process Mo/Fe is used as a catalyst.
- Co-Th alloy is used in Fischer Tropsch process in the synthesis of gasoline.
- Finely divided Ni, formed by reduction of the heated oxide in hydrogen is an extremely efficient catalyst in hydrogenation of ethene to ethane at 140 0
H2C=CH2 + H–H \(\frac{Δ}{Ni,140^0C}\)> H3C−CH3
- Commercially, hydrogenation with nickel as catalyst is used to convert inedible oils into solid fat for the production of margarine.
- In the contact process of industrial production of sulfuric acid; sulphur dioxide and oxygen from the air react reversibly over a solid catalyst of platinised asbestos.
2SO2 + O2 \(\underleftrightarrow{Platinised\,\,asbestos}\) 2SO3
- Carbon dioxide and hydrogen are formed by reaction of the carbon monoxide and steam at about 500 0C with an Fe-Cr catalyst.
CO + H2O (steam) \(\underleftrightarrow{Fe-Cr}\) CO2 + H2
Formation of interstitial compounds
Interstitial compounds : When small atoms like hydrogen, carbon or nitrogen are trapped in the interstitial spaces within the crystal lattice, the compounds formed are called interstitial compounds.
- Sometimes sulphides and oxides are also trapped in the crystal lattice of transition
- Presence of these elements in the crystal lattices of metals provide new properties to the metals
- Example : Steel and cast iron are interstitial compounds of carbon and iron. Due to presence of carbon, the malleability and ductility of iron is reduced while its tenacity
Properties of interstitial compounds
- They are hard and good conductors of heat and electricity.
- Their chemical properties are similar to the parent metal.
- Since metal-non-metal bonds in the interstitial compounds are stronger than metal-metal bonds in pure metals, the compounds have very high melting points, higher than the pure metals.
- Their densities are less than the parent metal.
- The metallic carbides are chemically inert and extremely hard as diamond.
- Hydrides of transition metals are used as powerful reducing agents.
- In these compounds. malleability and ductility are changed. For example steel and cast iron
Remember : Tungsten carbide is used for cutting tools and Iron carbide is used in manufacture of steel.
Formation of Alloys
Formation of alloys of transition metals :
- The transition metals form a large number of alloys among themselves, which are hard with high melting points.
- During alloy formation atoms of one metal are distributed randomly in the lattice of another metal.
- The metals with similar atomic radii and similar properties readily form alloys.
- These alloys have industrial importance.
- The alloys can be ferrous alloys or nonferrous alloys.
The transition metal alloys are classified into (i) Ferrous alloys (ii) Nonferrous alloys
(i) Ferrous alloys : In ferrous alloys, atoms of other elements are distributed randomly in crystal lattice of iron in the mixture. As the percentage of iron is more in these alloys, they are termed as ferrous alloys.
- Example : Nickel steel, chromium steel, stainless steel, (All steels have about 2% carbon)
(ii) Nonferrous alloys : These are formed by mixing atoms of transition metal other than iron with a non transition element.
- Example : Brass is an alloy of Cu and Zn. Bronze is an alloy of Cu and Sn.
Ferrous and nonferrous alloys are of industrial importance :
Uses of alloys :
| Alloy | Use |
| Bronze,
|
It is an alloy of copper and tin is tough, strong and corrosion resistant. It is used for making statues, medals and trophies. |
| Cupra-nickel,
|
It is an alloy of copper and nickel is used for making machinery parts of marine ships, boats. For example, marine condenser tubes. |
| Stainless steels | Used in the construction of the outer fuselage of ultra-high speed air craft. |
| Nichrome
|
It is an alloy of nickel and chromium in the ratio 80 : 20 has been developed specifically for gas turbine engines. |
| Titanium
|
It is withstand stress up to high temperatures and are used for ultrahigh speed flight, fire proof bulkheads and exhaust shrouds. |
PDF : Chapter-8-Transition and Inner transition Elements-Text Book
PDF : Chapter-8-Transition and Inner transition Elements- Notes
PDF : Chapter-8-Transition and Inner transition Elements- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-8-Elements of Groups 16, 17 and 18 – Online Notes
Next Chapter : Chapter-9-Coordination Compounds – Online Notes
We reply to valid query.