Chemical Thermodynamics
Maharashtra Board-Class-12-Chemistry-Chapter-4
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
Thermodynamics is concerned with the energy changes in physical and chemical transformations.
Drawbacks :
- It does not give information on the rates of physical or chemical changes.
- It does not explain mechanisms involved in physical and chemical processes.
Terms used in thermodynamics :
Term energy : The energy of a system is defined as its capacity to perform the work. A system with higher energy can perform more work. .
Different forms of energy and concept of interconversion of different forms of energy :
The energy of a system has many different forms as follows : .
- Kinetic energy which arises due to motion, like rotational, vibrational and translational.
- Potential energy which arises due to position and state of a matter. If depends upon the temperature of the system.
- Heat energy (or thermal energy) which is transferred from the hotter body to the colder body.
- Radiant energy which is associated with electro-magnetic or light radiation.
- Electrical energy produced in the galvanic cells.
- Chemical energy stored in chemical substances.
All these various forms of energy can be converted from one form to another without any loss, i.e. all these forms of energy are interconvertible
Examples :
- A body at very high level possesses higher potential energy. When it falls down, potential energy is converted into kinetic energy.
- Falling of water from high level is used to drive turbines converting potential energy into kinetic energy which is further converted into electrical energy,
- In galvanic cells, chemical energy is convened into electrical energy.
- In electrolytic cells, chemical energy is convened into electrical energy. But during interconversion, the energy can neither be created nor destroyed, and there is a conservation of energy.
System and surrounding :
System : A part of the universe under thermodynamic investigation is called the system.
Explanation :
- As such any portion of the universe under thermodynamic consideration is a system. The thermodynamic consideration involves; the study of thermodynamic parameters like; pressure, volume, temperature, energy, etc.
- The system may be very small or very large.
- The system is confined by a real or an imaginary boundary.
Surroundings : The remaining portion of the universe other than under thermodynamics study i.e., the system is called the surroundings.
Explanation :
- Surroundings represent a large stock of mass and energy and can be exchanged with the system when allowed.
- For a liquid in an open vessel, the surrounding atmosphere around it represents the surroundings.
Boundary : The wall or interface separating the system from its surrounding is called a boundary.
Explanation :
- This boundary may be either real or imaginary.
- Through this boundary, exchange of heat and matter between the system and surroundings can take place, e.g. when a liquid is placed in a beaker the Walls of beaker represent real boundaries while open portion of the beaker is imaginary boundary.
- Everything outside the boundary represents surroundings.

| Know This :
At the top of dam, water is stored in a reservoir. It has certain potential energy due to its height from ground level and its kinetic energy is negligible as it is not in motion. As the water starts to fall down through an outlet its potential energy decreases and kinetic energy increases due to the downward velocity. It means that potential energy of falling water is converted into kinetic energy. |
Types of systems :
- Open system
- Closed system
- Isolated system
- Homogeneous system
- Heterogeneous system.
Open system : It is defined as a system which can exchange both matter and energy with its surroundings.
- In this system, the total amount of matter does not remain constant.
- In this system, the total amount of energy does not remain constant.
- Example : Fig.(a) shows an open cup containing hot coffee placed in a room. We observe coffee cools down releasing heat to the surroundings. The water vapour from coffee simultaneously passes into surroundings. So that a system (coffee) which exchanges both energy and matter with the surroundings

Closed system : It is defined as a system which can exchange only energy but not the matter with its surroundings.
- In this system, the total amount of matter remains constant.
- In this system, the total amount of energy does not remain constant.
- Example : In Fig. (b), a cup containing hot coffee is covered with a saucer. Coffee cools down by giving away heat to the surroundings. The water vapour from coffee now does not pass into surroundings. So that a system that exchanges energy and not the matter with the surroundings.
Isolated system : It is defined as a system which can neither exchange energy nor matter with its surroundings.
- In this, the total amount of energy remains constant.
- Example : In Fig. (c), a cup containing hot coffee covered with a saucer is insulated from the surroundings. Coffee does not cool down. Moreover, there is no escape of water vapour into the surroundings. Such a system that does not allow exchange of either energy or matter with the surroundings.
In actual practice, perfectly isolated system is not possible. Universe represents an isolated system.
Q. Universe is an isolated system : Explain.
Ans. Universe represents an isolated system due to the following reasons :
- The total mass and energy of the universe remain constant.
- The universe has no boundary.
- The universe has no surroundings.
Homogeneous system : A system consisting of only one uniform phase is called a homogeneous system.
Explanation :
The properties of homogeneous system are uniform throughout the phase or system.
The homogeneous systems are :
- Solutions of miscible liquids (water and alcohol) or soluble solids in liquids, (NaCl in water), etc.
- Mixture of gases. H2 and N2, NH3 and H2, etc.
Heterogeneous system : A system consisting of two or more phases separated by interfacial boundaries is called a heterogeneous system.
Explanation : These systems are :
- Mixture of two or more immiscible liquids. E.g. Water and benzene.
- Solid in equilibrium with liquid. E. g. Ice ⇌ water.
- Liquid in equilibrium with vapour E.g. Water ⇌ vapour.
Properties of system :
The properties of a system are classified as
(A) Extensive property and (B) Intensive property
Extensive property : A property which depends on the amount of matter present in a system is called an extensive property.
Explanation :
- More the quantity (or amount) of the matter of the system, more is the magnitude of extensive property. e.g., mass, volume, heat, energy, enthalpy, etc.
- The extensive properties are additive.
Intensive property : A property which is independent of the amount of matter in a system is called intensive property.
Explanation :
- Intensive property is characteristic of the system. e.g., refractive index, density, viscosity, temperature, pressure, boiling point, melting point, freezing point of a pure liquid, surface tension, etc.
- The intensive properties are not additive
State function : The property which depends on the state of the system and independent of the path followed by the system to attain the final state is called a state function.
- Examples : pressure, volume, temperature, etc.

Here the term process means a physical or chemical change in a system on going from one state to another. This can be achieved by a number of paths by some operation.
Path functions : The properties which depend on the path of the process are called path functions. For example, work (W) and heat (Q).
Thermodynamic equilibrium : A system is said to have attained a state of thermodynamic equilibrium if there is no change in any thermodynamic functions or state functions like energy, pressure, volume, etc. with time.
- Consider a gas enclosed in a cylinder fitted with a movable piston. The gas has temperature T1, pressure P1 and volume V1. These state functions continue to be constant as long as piston is motionless, and no heat exchange takes place. This is an equilibrium state.
For a system to be in thermodynamic equilibrium, it has to attain following three types of equilibrium
- Thermal equilibrium
- Chemical equilibrium
- Mechanical equilibrium .
Process and its types :
Thermodynamic process : A transition from one equilibrium state to another equilibrium state is called a thermodynamic process.
The process is carried out by changing the state functions or thermodynamic variables like pressure, volume and temperature. During the process one or more properties of the system change.
Types of processes :
- Isothermal process
- Isobaric process
- Isochoric process
- Adiabatic process
- Reversible process
- Irreversible (spontaneous) process.
Isothermal process: It is defined as a process in which the temperature of the system remains constant throughout the change of a state of the system.
In this, ΔT = 0.
Features :
- In this process, the temperature at initial state, final state and throughout the process remains constant.
- In this process, system exchanges heat energy with its surroundings to maintain constant temperature. E.g., in case of exothermic process liberated heat is given to the surroundings and in case of endothermic process heat is absorbed from the surroundings so that temperature of the system remains constant and ΔT = 0.
- Isothermal process is carried out with a closed system.
- Internal energy (U) of the system remains constant, hence, ΔU = 0.
- In this process, pressure and volume of a gaseous system change.
- In this process, the system exchanges heat with the surroundings. Q ≠ 0 (Closed system)
- In this process, the system is not thermally isolated.
- In this process, Q = − W as ΔU = 0.
- ΔH = 0.
Isobaric process : It is defined as a process which is carried out at constant pressure. Hence, ΔP = 0.
Features :
- In this process, the volume (of gaseous system) changes against a constant pressure.
- Since the external atmospheric pressure remains always constant, all the processes carried out in open vessels, or in the laboratory are isobaric processes.
- In this volume and temperature change
- Internal energy of a system changes, hence ΔU ≠ 0.
- In this process, the system does not exchange heat with the surroundings. Q = 0 (Isolated system)
- In this process, the system is thermally isolated.
- In this process, W = ΔU,
- ΔH ≠ 0
Isochoric process : It is defined as a process which is carried out at constant volume of the system.
Features :
- In this process, temperature and pressure of the system change but volume remains constant.
- Since ΔV = 0,no mechanical work is performed.
- In this internal energy (U) of the system changes.
- Example of this process in cooking takes place in a pressure cooker.
Adiabatic process : It is defined as a process in which there is no exchange of heat energy between the system and its surroundings. Hence, Q = 0.
Features :
- An adiabatic process is carried out in an isolated system.
- In this process, temperature and internal energy of a system change, ΔT ≠ 0, ΔU ≠ 0.
- During expansion, temperature and energy decrease and during compression, temperature and energy increase.
- If the process is exothermic, the temperature rises and if the process is endothermic the temperature decreases in the adiabatic process.
Reversible process : A process carried out in such a manner that at every stage, the driving force in only infinitesimally greater than the opposing force and it can be reversed by an infinitesimal increases in force and the system exists in equilibrium with its surroundings throughout, is called a reversible process.
Features :
- This is a hypothetical process.
- Driving force is infinitesimally greater than this opposing force throughout the change.
- The process can be reversed at any point by making infinitesimal changes in the conditions.
- The process takes place infinitesimally slowly involving infinite number of steps.
- At the end of every step of the process, the system attains mechanical equilibrium, hence throughout the process, the system exists in temperature-pressure equilibrium with its surroundings.
- In this process, maximum work is obtained.
- Temperature remains constant throughout the isothermal reversible process.
Irreversible process : It is defined as the unidirectional process which proceeds in a definite direction and cannot be reversed at any stage and in which driving force and opposing force differ in a large magnitude. It is also called a spontaneous process.
Features :
- It takes place without the aid of external agency.
- All irreversible processes are spontaneous and takes finite time for completion.
- All natural processes are irreversible processes.
- The thermodynamic equilibrium is attained only at the end of the process.
- They are real processes and are not hypothetical.
- The opposing force is significantly less than the driving force.
- It is a practical or real and spontaneous process.
- Work derived from such a process is always less than the maximum work.
Examples :
- Flow of heat from a matter at higher temperature to a matter at lower temperature.
- Flow of a gas from higher to lower pressure.
- Flow of water from higher level to lower level.
- Flow of a solvent into a solution through a semipermeable membrane due to osmosis.
- Flow of electricity from higher potential terminal to lower potential terminal.
Nature of heat and work :
Nature of work (W) : In mechanics the work is defined as the energy by which body is displaced through a distance d with an application of force f.
∴ W = f x d
If area is A = d2 and volume V = d3 then,
PV = \(\frac{f}{A}×d^3\) = \(\frac{f}{d^2}×d^3\) = f x d = W
∴ the term PV represents the pressure-volume work.
In thermodynamics the type of work involved is pressure-volume or PV work, that is, work is done when the system (gas) expands or contracts against the external opposing force.
Process of expansion :
Consider an ideal cylinder fitted with a piston and filled with H2O2(l).
2H2O2(l) → 2H2O(l) + O2(l)

The oxygen gas produced pushes the piston upwards lifting the mass. Thus, the system performs the work on the surroundings and loses energy by expansion. In this work is done by the system.
Process of compression :
Consider an ideal cylinder fitted with a piston and containing gaseous NH3(g) and HCl(g).
NH3(g) + HCl(g) → NH4Cl(s)

As the reaction proceeds, due to consumption of gases, the volume decreases and there is work due to compression. In this work is done on the system by surroundings and the system gains energy.
Nature of heat (Q) : Heat is a form of energy by which the system exchanges energy with its surroundings. When the system and its surroundings are at different temperatures heat either flows in or let out of the system.
Sign conventions of W and Q :

(A) For expansion, work is done by the system hence,
Q = −ve and W = −ve
(B) For compression, work is done on the system hence
Q = −ve and W = +ve
Expression for pressure-volume (PV) work
Consider a certain amount of gas at constant pressure P is enclosed in a cylinder fitted with frictionless, rigid movable piston of area A. Let volume of the gas be V1 at temperature T.
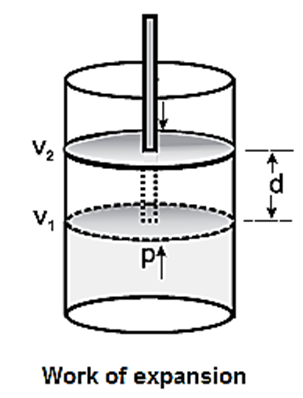
As the gas expands, it pushes the piston upward through a distance d against external force F pushing the surroundings.
The work done by the gas is,
W = opposing force x distance
= − F x d
− ve sign indicates the lowering of energy of the system during expansion.
If a is the cross section area of the cylinder or piston, then,
W = \(-\frac{f}{a}×d×x\)
Now the pressure IS Pex = F/a
while volume change is, ΔV = d x a
∴ W = −Pex x ΔV
If during the expansion, the volume changes from V1 and V2 then, ΔV = V2 − V1
∴ W = − Pex(V2 − V1)
During compression, the work W is + ve, since the energy of the system is increased,
W = +Pex(V2 − V1)
Explanation of +W and −W :
- During expansion V2 > V1. The work is said to be performed by the system on the surroundings. This results in the decrease in the (work) energy of the system. Hence the work is negative, i.e. W is — ve.
- During compression, V2 < V1. The work is said to be performed on the system by the surroundings. This results in the increase in the (work) energy of the system. Hence the work is positive, i.e. W is +ve.
Free expansion : A free expansion means expansion against zero opposing force.
Such expansion occurs in vacuum. The work done by a system during such expansion is given by Eq. W = − Pex ΔV.
When a gas expands against an external pressure Pex, changing the volume from V1 to V2, the work obtained is given by
W = − Pex (V2 − V1).
Hence the work is performed by the system when it experiences the opposing force or pressure.
Greater the opposing force, more is the work.
In free expansion, the gas expands in vaccum (where it does not experience opposing force, (P = 0). Since external pressure is zero, no work is obtained.
W = −Pex(V2 − V1)
= −0 x (V2 − V1) = 0
Since during expansion in vacuum no energy is expended, it is called free expansion.
Units of energy and work
1 J = 1 kg m2 s−2 = 1 Pa m3
1 Pa = 1 kg m−1 s−2
From to Eq., W = −Pex
ΔV, if pressure is expressed in bar and ΔV in dm3, then the work has the units of bar-dm3.
1 bar = 105 Pa = 105 kg m−1 s−2
1 dm3 bar = dm3 × 105 kg m−1 s−2
= m3 × 10−3 × 105 kg m−1 s−2
= 100 kg m2 s−2 = 100 J
1 cal = 4.184 J
Concept of maximum work : Eq. W = −Pex shows the amount of work performed by a system is governed by the opposing force (Pex).
- Larger the opposing force more work is done by the system to overcome it.
- Thus when the opposing force (Pex) becomes greater than the driving force (P) the process gets reversed. Since the opposing force cannot be greater than the driving force it should be the maximum.
- The maximum work is obtained from the change which is thermodynamically reversible.
Expression for the work obtained in an isothermal reversible expansion of an ideal gas. OR Expression for maximum work :
Consider n moles of an ideal gas enclosed in an ideal cylinder fitted with a massless and frictionless movable rigid piston.
Let V be the volume of the gas at a pressure P and a temperature T.
If in an infinitesimal change pressure changes from P to P — dP and volume increases from V to V + dV. Then the work obtained is,
dW= − (P − dP)dV
= − PdV + dPdV
Since dP. dV is negligibly small relative to PdV
dW = − PdV
Let the state of the system change from A(P1, V1) to B (P2, V2) isothermally and reversibly, at temperature T involving number of infinitesimal steps.

Then the total work or maximum work in the process is obtained by integrating above equation.
Wmax = \(\int_{A}^{B}\)dW =\(\int_{A}^{B}\)−PdV
‘.’ PV = nRT
∴ P = nRT/V
Wmax = \(\int_{V_1}^{V_2}\)−nRT\(\frac{dV}{V}\)
= −nRT\(\int_{V_1}^{V_2} \frac{dV}{V}\)
= −nRT(lnV2 − lnV1)
= −nRT loge \(\frac{V_2}{V_1}\)
∴ Wmax = −2.303 nRT log10 \(\frac{V_2}{V_1}\)
At constant temperature,
‘.’ P1 x V1 = P2 x V2
\(\frac{V_2}{V_1}=\frac{P_1}{P_2}\)
Wmax = −2.303 nRT log10 \(\frac{P_1}{P_2}\)
Characteristics of maximum work :
- The process is carried out at constant temperature,
- During the complete process, driving force is infinitesimally greater than opposing force.
- Throughout the process, the system exists in equilibrium with its surroundings.
- The work obtained is maximum. This is given by,
Wmax = −2.303 nRT log10 \(\frac{V_2}{V_1}\)
OR
Wmax = −2.303 nRT log10 \(\frac{P_1}{P_2}\)
where n, P, V and T represent number of moles, pressure, volume and
temperature respectively.
- ΔU = 0, ΔH = 0.
- The heat absorbed in reversible manner
Qrev, is completely converted into work
Qrev = − Wmax
Hence work obtained is maximum.
Internal energy (U)
Internal energy : It is defined as the total energy constituting potential energy and kinetic energy of the molecules present in the system.
Explanation :
- The internal energy of a system is a state function and thermodynamic function. It is denoted by U.
- Its value depends on the state of a system.
- The change in internal energy (A U) depends only on the initial state and the final state of the system. ΔU = U2 − U1
- It is an extensive property of the system.
- It has same unit as heat and work.
- Total internal energy U of the system is,
Total energy = Potential energy + Kinetic energy
Examples - How to determine ΔU.
- 25 kJ of heat supplied to the system. It would be added to internal energy of the system and ΔU = +25 kJ.
- If 20 kJ of work is done on the system, it is added to internal energy of the system. Consequently, ΔU = + 20 kJ.
- Suppose a system releases 10 kJ of heat and performs 15 kJ of work on the surroundings. These quantities are removed from internal energy of the system and ΔU = − 25 kJ
First law of thermodynamics : First law of thermodynamics is simply the conservation of energy.
According to this law the total energy of a system and surroundings remains constant when the system changes from an initial state to final state.
The law is stated in different ways as follows :
- Energy can neither be created nor destroyed, however, it may be converted from one form into another.
- Whenever, a quantity of one kind of energy is consumed or disappears, an equivalent amount of another kind of energy appears.
- The total mass and energy of an isolated system remain constant, although there may be inter-conservation of energy from one form to another.
- The total energy of the universe remains constant.
Formulation of first law of thermodynamics (mathematical equation for the first law of thermodynamics) :
- The first law of thermodynamics is based on the principle of conservation of energy.
- If Q is the heat absorbed by the system and if W is the work done by surroundings on the system then the internal energy of the system will increase by ΔU.
- From the conservation of energy we can write, Increase in internal energy of the system = Quantity of heat absorbed by the system + Work done on the system
ΔU = Q + W
- For an infinitesimal change,
dU = dQ + dW
First law of thermodynamics for various processes :
Isothermal process : This is a process which is carried out at constant temperature. Since internal energy, U of the system depends on temperature there is no change in the internal energy U of the system. Hence ΔU = 0.
By first law of thermodynamics,
ΔU = Q + W
∴ 0 = Q + W
∴ Q = −W Or W = −Q.
- Hence in expansion, the heat absorbed by the system is entirely converted into work on the surroundings.
- In compression, the work done on the system is converted into heat which is transferred to the surroundings by the system, keeping temperature constant.
Isobaric process : In this, throughout the process pressure remains constant. Hence the system performs the work of expansion due to volume change ΔV.
‘W = − Pext x ΔV
Let QP, be the heat absorbed by the system at constant pressure.
By first law of thermodynamics,
ΔU = QP + W
∴. ΔU = QP − Pext x ΔV
Or QP = ΔU + Pext x ΔV
In this process, the heat absorbed QP is used to increase the internal energy (ΔU) of the system.
Adiabatic process : In this process, the system does not exchange heat, Q with its surroundings.
Q = 0.
Since by first law of thermodynamics,
ΔU = Q + W
∴ ΔU = Wad
Hence,
- The increase in internal energy ΔU is due to the work done on the system by surroundings. This results in increase in energy and temperature of the system.
- If the work is done by the system on surroundings, like expansion, then there is a decrease in internal energy ( −ΔU) and temperature of the system decreases.
Remember...
(i) Heat absorbed = + Q Heat evolved = − Q (ii) Internal energy change : Increase in energy = + ΔU Decrease in energy = − ΔU (iii) Work done by the system = − W Work done on the system = + W |
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-3- Ionic Equilibria – Online Notes
Next Chapter : Chapter-5-Electrochemistry – Online Notes
We reply to valid query.