Introduction to Polymer Chemistry
Maharashtra Board-Class-12-Chemistry-Chapter-15
Notes
|
Topics to be Learn :
|
Introduction :
Polymers are high molecular mass macromolecules (103-107 u) and consists of repeating units of monomers.
- Staudinger was awarded the Nobel Prize in Chemistry in 1953 for his work establishing the macromolecular nature of polymers.
- Natta was awarded the Nobel Prize in Chemistry in 1963 for discovering stereospecific regularity in vinyl polymers.
- Flory was awarded the Nobel Prize in 1974 for revealing the three-step mechanism of chain-reaction in polymerization, which included initiation, propagation, and termination.
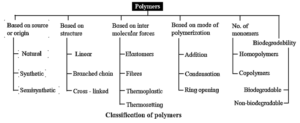
Classification of polymers :
Polymers are classified on the basis of their source, chemical structures, mode of
polymerization, molecular forces, type of monomers and biodegradability.
Classification of polymers on the basis of source or origin :
Based on the source polymers are classified as natural, semisynthetic and synthetic polymers.
(1) Natural polymers : These polymers are obtained either from plants or animals. Therefore they are further divided into two types : (i) Plant polymers (i) Animal polymers.
- Plant polymers : These are obtained from plants. For example, cotton and linen are obtained from cotton plant and flax plant respectively. Natural rubber is manufactured from the latex obtained from bark of rubber trees.
- Animal polymers : These are derived from animal sources. For example, wool is obtained from hair of sheep. Silk is obtained from silkworm.
(2) Semisynthetic polymers : The fibres obtained by giving special chemical treatment to natural fibres (cellulose) and further regenerated are called semisynthetic polymers e.g., acetate rayon, viscose rayon, cuprammonium silk.
(3) Synthetic polymers : The man made fibres prepared by polymerization of one monomer or copolymerization of two or more monomers are called synthetic polymers. They are further divided into three subtypes : (i) Fibres (ii) Synthetic rubbers (iii) Plastics. e.g., nylon, terylene, Buna-S, polythene, etc.
Classification of polymers on the basis of structure :
Based on structure polymers are classified as linear chain polymers, branched chain polymers and network or cross linked polymers.
(a) Linear chain polymers : When the monomer molecules are joined together in a linear arrangement, the resulting polymer is straight chain or long chain polymer. e.g., polythene, PVC.
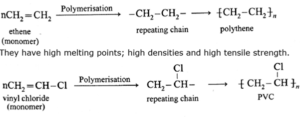
(b) Branched chain polymers : These polymers consist of long and straight chain with smaller side chains give rise to branched chain polymers. They have low density. They have lower melting points and tensile strength. Polypropylene having methyl groups as branches.
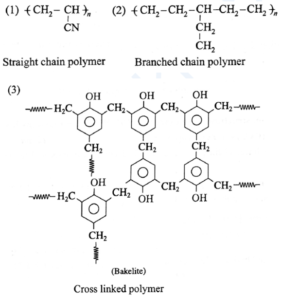
(c) Network or cross linked polymers : These polymers consist cross linking of chains by strong covalent bonds leading to network like structure. Cross linking results from polyfunctional monomers. e.g., melamine, bakelite, vulcanization of rubber. These polymers are hard rigid and brittle.
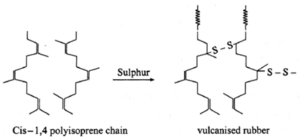
Examples :
Classification of polymers on the basis of mode of polymerization :
Polymerization is the fundamental process by which low molecular mass compounds are converted into high molecular weight compounds by linking together of repeating structural units with covalent bonds.

There are three modes of polymerization depending upon the types of reactions taking place between the monomers.
- Addition polymerization (or chain growth polymerization)
- Condensation polymerization (or step growth polymerization)
- Ring opening polymerization
(i) Addition polymerization or chain growth polymerization : It is a process of polymers by the repeated addition of a large number of monomers is called addition polymerization (like alkenes) without loss of any small molecules.
- The repeating unit of an addition polymer has the same elemental composition as that of original momer.
- Addition polymerization produces high molecular mass polymeric materials free radical mechanism is most common in addition polymerization.
Example : Formation of polyethylene from ethylene is well known example of addition polymerization. It is a chain growth polymerization.
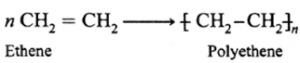
Addition polymers : PVC, polythene, poly acrylonitrile, polystyrene.
Free radical mechanism : Free radical mechanism is most common in addition polymerisation. It is also called chain reaction which involves three distinct steps chain initiation, chain propagation and chain termination.
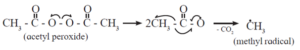
Step 1 : Chain initiation : The chain reaction is initiated by a free radical.
An initiator (catalyst) such as benzoyl peroxide, acetyl peroxide, tert-butyl peroxide, etc. can be used to produce free radical. For example acetyl peroxide generates methyl radical as shown below :
The free radical (say R) so formed attaches itself to the olefin (vinyl monomer) and produces a new radical, made up of two parts, namely, the attached radical and the monomer unit.
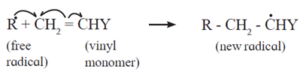
Step 2 : Chain propagation : The new radical formed in the initiation step reacts with another molecule of vinyl monomer, forming another still bigger sized radical, which in turn reacts with another monomer molecule. The repetition of this sequence takes place very rapidly. It is called chain propagation.
![]()
This step is very rapid and leads to high molecular mass radical.
Step 3 : Chain termination : Ultimately, at some stage, termination of the growing chain takes place. It may occur by several processes. One mode of termination is by combination of two growing chain radicals.
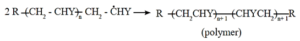
(ii) Condensation polymerization or step growth polymerization : The process of formation of polymers from polyfunctional monomers with the elimination of some small molecules such as water, hydrochloric acid, methanol, ammonia is called condensation polymerization.
Example : The formation of terylene, a polyester polymer, from ethylene glycol and terephthalic acid. In this reaction an alcoholic OH group in ethylene glycol condenses with a carboxyl group in terephthalic acid by eliminating a water molecule to form an ester linkage.
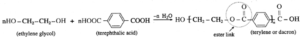
Condensation polymerization is a step growth polymerization.
Condensation polymers : Polyester.
(iii) Ring opening polymerization : The process of formation of polymers from cyclic compounds (like lactams, cyclic ethers, etc.) by ring opening is called ring opening polymerization.
Example : Polymerization of e-caprolactam.
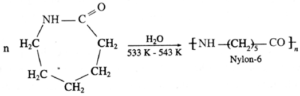
Ring opening polymerization proceeds by addition of a single monomer unit to the growing chain molecules. It is a step growth polymerization.
Ring opening polymers : Cyclic ethers
Classification of polymers on the basis of intermolecular forces :
Mechanical properties of polymers such as tensile strength, toughness, elasticity differ widely depending upon the intermolecular forces. Polymers are classified into various categories on the basis of intermolecular forces as follows.
(i) Elastomers :
- Weak van der Waals type of intermolecular forces of attraction between the polymer chains are observed in elastomers, which permit the polymer to be stretched.
- When polymer is stretched, the polymer chain stretches and when the strain is relieved the chain returns to its original position. Thus, polymers have elastic character like rubber are called elastomers.
- Elastomers are soft and stretchy and used in making rubber bands.
- Examples : Neoprene, vulcanized rubber, buna-S, buna-N.
(ii) Fibres :
- Polymeric solids which form threads are called fibres.
- The fibres possess high tensile strength which is a property to have resistance to breaking under tension.
- This characteristic is due to the strong intermolecular forces like hydrogen bonding and strong dipole-dipole forces.
- Due to these strong intermolecular forces the fibres are crystalline in nature.
- Examples : Polyamides (nylon 6, 6), polyesters (terylene), etc.
(iii) Thermoplastic polymers :
- These polymer possess moderately strong intermolecular forces of attraction between those of elastomers and fibres.
- These polymers are called thermoplastic because they become soft on heating and hard on cooling.
- They are either linear or branched chain polymers. They can be remoulded and recycled.
- Examples : Polyethene, PVC, polystyrene, PET.
(iv) Thermosetting polymers :
- These polymers are cross linked or branched molecules and are rigid polymers.
- During their formation they have property of being shaped on heating, but they get hardened while hot.
- Once hardened these become infusible, cannot be softened by heating and therefore, cannot be remoulded and recycled.
- This shows extensive cross linking by covalent bonds formed in the moulds during hardening/setting process while hot.
- Examples : Bakelite, urea formaldehyde resin, phenol formaldehyde.
Classification of polymers on the basis of type of different monomers :
Polymers are divided into two classes :
(i) Homopolymers : The polymers which have only one type of repeating unit are called homopolymers.
- Usually they are formed from a single monomer. In some cases the repeating unit is formed by condensation of two distinct monomers.
- Examples : polythene, polypropene, Nylon 6, polyacrylonitrile, Nylon 6, 6.
(ii) Copolymers : The polymers which have two or more types of repeating units are called copolymer.
- They are formed by polymerization of two or more different types of monomers in presence of each other.
- The different monomer units are randomly sequenced in the copolymer.
- Examples : Buna-S, Buna-N
Classification of polymers on the basis of biodegradability :
Based on biodegradability, polymers are classified as biodegradable polymer and nonbiodegradable polymers.
(i) Biodegradable polymers :
- Polymers which are affected by microbes or disintegrate by themselves after a certain period of time due to environmental degradation are called biodegradable polymers.
- Biodegradable polymers and their graded products do not cause any serious effects on the environment.
- Examples : PHBV i.e., Polyhydroxy butyrate-CO-β-hydroxy valerate Dextron, Nylon—2—nylon—6.
(ii) Non biodegradable polymers :
- Synthetic polymers do not disintegrate by themselves after a certain period or not affected by microbes, are called nonbiodegradable polymers.
- These are in the form of waste material which stays in the environment for very long time and cause pollution hazards.
- Examples : Bakelite, Nylon, Terylene.
All the classes of polymers in the form of a tree diagram :
Some important polymers :
Rubber : Elastomers are popularly known as rubbers.
Examples : Balloons, shoesoles, tyres, surgeon's gloves, garden hose, etc. are made from elastomeric material or rubber.
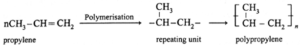
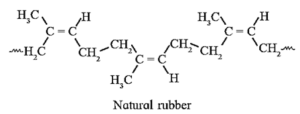
Natural rubber : Natural rubber is a high molecular mass linear polymer of isoprene (2-methyl-1, 3-butadiene).
Natural rubber is manufactured from rubber latex obtained from the rubber tree in the form of colloidal suspension.
Reaction involved in the formation of natural rubber by the process of addition polymerization is as follows :

Properties of Natural rubber :
- Polyisoprene molecule has cis configuration of the C = C double bond. It consists of various chains held together by weak van der Waals forces and has coiled structure.
- It can be stretched like a spring and exhibits elastic property.
- Its molecular mass varies from 130,000 u to 340,000 u.
Vulcanization of rubber :
Vulcanisation is the technique of introducing a network of cross links into an elastomer. It can also be defined as the process of heating natural rubber with sulphur to increase the tensile strength, toughness, and elasticity of natural rubber.
- The tensile strength, toughness and elasticity of natural rubber can be increased by adding 3 to 5% sulphur and heating at 100—150°C, resulting in cross linking of cis—1, 4—polypropene chains through disulphide bonds, (-S—S-).
- Rubber made with 20—30% sulphur is hard, 3 to 10% sulphur is little harder and is used in making tyres and 1 to 3% sulphur is used in making rubber bands.
Polythene :
Polythene is the simplest and most commonly used hydrocarbon thermoplastic and has following structure.
[CH2 − CH2]n
The IUPAC name of polyethylene is polythene. Polythene is of two kinds, namely low density polythene (LDP) and high density polythene (HDP) .
Low density polyethylene (LDP) : LDP is obtained by polymerization of ethylene under high pressure (1000-2000 atm) and temperature (350-570 K) in presence of traces of O2 or peroxide as initiator.
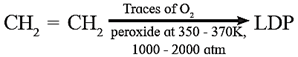
The mechanism of this reaction involves free radical addition and H-atom abstraction. The latter results in branching. As a result the chains are loosely held and the polymer has low density.
Properties of LDP :
- LDP films are extremely flexible, but tough chemically inert and moisture resistant.
- Itis poor conductor of electricity with melting point 110 °C.
Uses of LDP :
- LDP is mainly used in preparation of pipes for agriculture, irrigation, domestic water line connections as well as insulation to electric cables.
- It is also used in submarine cable insulation.
- It is used in producing extruded films, sheets, mainly for packaging and household uses like in preparation of squeeze bottles, attractive containers, etc.
High density polyethylene( HDP) : It is a linear polymer with high density due to close packing.
![]()
HDP is obtained by polymerization of ethene in presence of Zieglar-Natta catalyst which is a combination of triethyl aluminium with titanium tetrachloride at a temperature of 333K to 343K and a pressure of 6-7 atm.
Properties of HDP :
- HDP is crystalline, melting point in the range of 144—150 °C.
- It is much stiffer than LDP and has high tensile strength and hardness.
- It is more resistant to chemicals than LDP.
Uses of HDP :
- HDP is used in manufacture of toys and other household articles like buckets, dustbins, bottles, pipes, etc.
- Ttis used to prepare laboratory wares and other objects where high tensile strength and stiffness is required.
Teflon :
- Teflon is polytetrafluoroethylene.
- It is an addition polymer of tetrafluoroethene.
- The monomer used in preparation of teflon is tetrafluoroethylene, (CF2=CF2) which is a gas at room temperature.
- Tetrafluoroethylene is polymerized by using free radical initiators such as hydrogen peroxide or ammonium persulphate at high pressure.
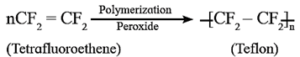
Properties :
- Telflon is tough, chemically inert and heat resistant and resists corrosive attacks of acids and bases.
- C—F bond is very difficult to break and remains unaffected by corrosive alkali, organic solvents.
Uses : Telflon is used in making blankets, carpets, non-stick cookware, oil seals, gaskets, etc.
Polyacrylonitrile :
Polyacrylonitrile is prepared by addition polymerization of acrylonitrile by using peroxide initiator.
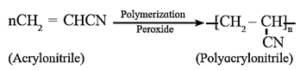
Uses : Polyacrylonitrile resembles wool and is used as wool substitute and for making orlon or acrilan.
- Orlon is used to make blankets, shawls, sweat shirts, sweaters.
Polyamide polymers :
Polyamide polymers are generally known as nylons. It is obtained by the condensation polymerization between dicarboxylic acids and diamines, the self-condensation of an amino acid or by the ring opening polymerization of lactams.
Polyamides contain -CO-NH– groups as the inter unit linkages. Two important polyamide polymers are nylon 6,6 and nylon 6.
(a) Nylon-6, 6 : Nylon-6, 6 is a linear polyamide polymer formed by the condensation polymerisation reaction. The monomers used in the preparation of Nylon—6, 6 are :
- Adipic acid : HOOC—(CH2)4—COOH
- Hexamethylene diamine : H2N—(CH2)6—NH2
When equimolar aqueous solutions of adipic acid and hexamethylene diamine are mixed and heated, there is neutralization to form a nylon salt. During polymerisation at 553 K nylon salt loses a water molecule to form nylon 6, 6 polymer. Both monomers (hexamethylene diamine and adipic acid) contain six carbon atoms each, hence the polymer is termed as Nylon—6,6.
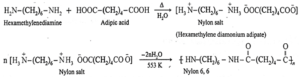
Properties : Nylon 6,6 is high molecular mass (12000-50000 u) linear condensation polymer. It possesses high tensile strength. It does not soak in water.
Uses : It is used for making sheets, bristles for brushes, surgical sutures, textile fabrics, etc.
(b) Nylon 6 : When epsilon (ε)-caprolactam is heated with water at high temperature it undergoes ring opening polymerization to give the polyamide polymer called nylon 6.
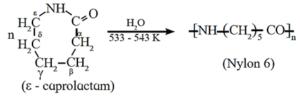
The name nylon-6 is given on the basis of six carbon atoms present in the monomer unit. Nylon-6 has high tensile strength and luster, nylon-6 fibres are used for manufacture of tyre cords, fabrics and ropes.
Polyesters :
- The polyester polymers have ester linkage joining the repeating units.
- Commercially the most important polyester fibre is 'terylene' (also called dacron).
- Terylene is formed by the polymerization of terephthalic acid and ethylene glycol.
- Terylene is obtained by condensation polymerization of ethylene glycol and terephthalic acid in presence of catalyst zinc acetate and antimony trioxide at high temperature.
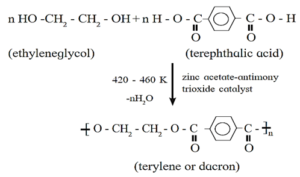
Properties :
- Terylene has relatively high melting point (265 °C)
- Itis resistant to chemicals and water.
Uses :
- It is used for making wrinkle free fabrics by blending with cotton (terycot) and wool (terywool), and also as glass reinforcing materials in safety helmets.
- PET is the most common thermoplastic which is another trade name of the polyester polyethyleneterephthalate. It is used for making many articles like bottles, jams, packaging containers.
Examples :
(i) structure of repeating unit formed by polymerisation of glycerol and DMT.
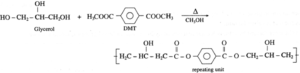
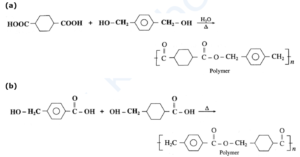
(ii) structures of polymers formed using the monomers.
Phenol - formaldehyde and related polymers :
(a) Bakelite : The ortho and para substituted phenols can undergo polymerization to produce a cross linked polymer known as bakelite.
The raw material or monomers used to prepare bakelite are o-hydroxymethyl phenol and formaldehyde (HCHO).
Phenol and formaldehyde react in presence of acid or base catalyst to form thermosetting/moulding powder (novolac) in two stages.
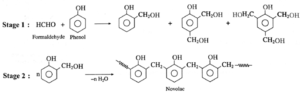
In the third stage, various articles are shaped from novolac by putting it in appropriate moulds and heating at high temperature (1380C to 1760C) and at high pressure forms rigid polymeric material called bakelite. Bakelite is insoluble and infusible and has high tensile strength.
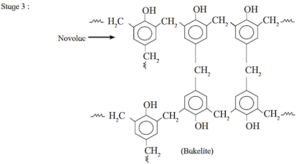
Uses : Bakelite is used in making articles like telephone instrument, kitchenware, electric insulators frying pans, etc.
(b) Melamine-formaldehyde polymer : The monomers formaldehyde and melamine undergo condensation polymerisation to form cross linked melamine formaldehyde.
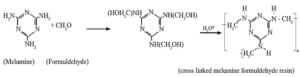
Uses : It is used in making crockeries, decorative table tops like formica and plastic dinner-ware.
Buna-S rubber :
Its trade name is SBR (Styrene-butadiene rubber) Buna-S is a copolymer of styrene and 1, 3-butadiene. When 75 parts of butadiene and 25 parts of styrene subjected to addition polymerization by the action of sodium, Buna-S is obtained.
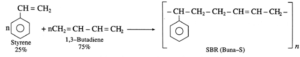
Uses : Buna-S is superior to natural rubber with regard to mechanical strength and has abrasion resistance. Hence, it is used in tyre industry.
Neoprene :
Neoprene, a synthetic rubber, is a condensation polymer of chloroprene (2-chloro-1, 3-hutadiene), Chloroprene polymerizes rapidly in presence of oxygen.
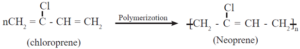
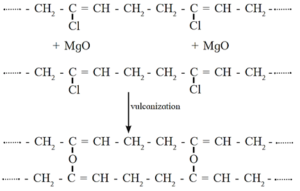
Vulcanization of neoprene takes place in presence of magnesium oxide.
Uses : Neoprene is resistant to petroleum, vegetable oils, light as well as heat. It is used in making hose pipes for transport of gasoline and making gaskets. It is used for manufacturing insulator cable, jackets, belts for power transmission and conveying.
Viscose rayon :
Viscose rayon is a semisynthetic fibre. It is a regenerated cellulose. The molecular formula of cellulose is (C6H10O5)n. A modified representation of the molecular formula of cellulose Cell-OH is used in the reactions involved in viscose formation.
Formation of viscose rayon :
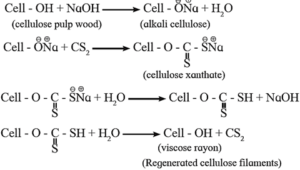
Cellulose (wood pulp) is treated with the concentrated NaOH to form alkali cellulose. It is then converted to xanthate by treating with CS2. Further xanthate is mixed with dilute NaOH to form viscose solution which is extruted through spinnerates of spinning machine into acid bath, when regenerated cellulose fibres precipitate. i.e. viscose rayon.
Molecular mass and degree of polymerization of polymers :
- Molecular mass of a polymer is an average of the molecular masses of the constituent molecules.
- Molecular mass of polymer depends upon the degree of polymerization (DP). DP is the number of monomer units in a polymer molecule.
Know This : Nylon 6 has strong intermolecular hydrogen bonding as inter molecular forces. On the other hand polythene chains have only weak van der Waals forces as intermolecular interaction. Because of the stronger intermolecular forces the critical DP is lower for nylon 6 than polythene.
Biodegradable polymers :
These polymers contain functional groups similar to those in biopolymers such as proteins. Aliphatic polyesters are also an important class of biodegradable polymers.
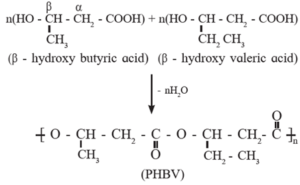
PHBV : It is a copolymer. The monomers β-hydroxy butyric acid (3-hydroxy butanoic acid) and β-hydroxy valeric acid (3-hydroxy pentanoic acid) undergo polymerization to form PHBV polymer. It has an ester linkage. Hydroxyl group of one monomer forms ester link by reactine with carboxyl group of the other. Thus PHBV is an aliphatic polyester i.e. poly β-hydroxybutyrate-co- β-hydroxy valerate (PHBV). It is a biodegradable polymer.
Nylon 2 - nylon 6 : Nylon 2-nylon 6 is a polyamide copolymer of two amino acids, namely, glycine and e - amino caproic acid. It is a biodegradable polymer.
Commercially important polymers :
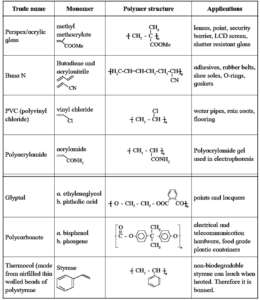
PDF : Class-12-Chemistry-Chapter-15-Introduction to Polymer Chemistry-Text Book
PDF : Class-12-Chemistry-Chapter-15-Introduction to Polymer Chemistry- Notes
PDF : Class-12-Chemistry-Chapter-15-Introduction to Polymer Chemistry- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 14- Biomolecules – Online Notes
Next Chapter : Chapter-16-Green Chemistry and Nano chemistry– Online Notes
We reply to valid query.