Modern Periodic Table
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -7
Solutions
Question 1. Explain the following
(A) The elements Li, B, C, Be and N have the electronegativities 1.0, 2.0, 1.5 and 3.0, respectively on the Pauling scale.
(B) The atomic radii of Cl, I and Br are 99, 133 and 114 pm, respectively.
Cl (99 pm) < Br (114 pm) < I (133 pm) Hence, the atomic radii of Cl, I, and Br are 99, 133, and 114 pm, respectively.
(C) The ionic radii of F- and Na+ are 133 and 98 pm, respectively.
(D) 13Al is a metal, 14Si is a metalloid and 15P is a nonmetal.
(E) Cu forms coloured salts while Zn forms colourless salts.
Cu : 1s2 2s2 2p6 3s2 3p6 4s1 3d10 Zn : 1s2 2s2 2p6 3s2 3p6 4s2 3d10 Since, Zn has completely filled s and d orbitals, it does not form coloured ions. Cu has incomplete 4s subshell hence it forms coloured ions.
Question 2. Write the outer electronic configuration of the following using orbital notation method. Justify.
(A) Ge (belongs to period 4 and group 14)
Ge is in period 4 and group 14. Therefore, its outermost electronic configuration is 4s2 4p2. In the fourth period 3d subshell is filled before 4p. Hence, the electronic configuration is 32Ge : 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2.
(B) Po (belongs to period 6 and group 16)
Po is in period 6 and group 16. Therefore, its outermost electronic configuration is 6s2 6p4. In sixth period, 4f and 5d subshells are filled up before 6p. Hence, electronic configuration of Po is 84Po : 1s2 2s22p6 3s23p6 4s23d104p6 5s24d10 5p6 6s2 4f14 5d10 6p4.
(C) Cu (belongs to period 4 and group 11)
Cu is in Period 4 and group 11. Therefore, its outermost electronic configuration is 4s2. In fourth period, 3d subshell is filled up and outer electronic configuration should be 3d9 4s2. Half-filled or completely filled subshell is more stable. Hence, the electronic configuration of Cu is 29Cu : 1s2 2s22p6 3s23p6 3d10 4s1
Question 3. Answer the following
(A) La belongs to group 3 while Hg belongs to group 12 and both belong to period 6 of the periodic table. Write down the general outer electronic configuration of the ten elements from La to Hg together using orbital notation method.
72Hf 73Ta 74W 75Re 76Os 77Ir 78Pt 79Au 80Hg [Xe] 4f14 5d2 6s2 [Xe] 4f14 5d3 6s2 [Xe] 4f14 5d4 6s2 [Xe] 4f14 5d5 6s2 [Xe] 4f14 5d6 6s2 [Xe] 4f14 5d7 6s2 [Xe] 4f14 5d9 6s1 [Xe] 4f14 5d10 6s1 [Xe] 4f14 5d10 6s2
Element
General outer electronic configuration orbital notation
57La
[Xe] 5d1 6s2
(B) Ionization enthalpy of Li is 520 kJ mol-1 while that of F is 1681 kJ mol-1. Explain.
(C) Explain the screening effect with a suitable example.
Screening effect : The inner shell electrons in an atom screen or shield the outermost valence electrons from the nuclear attraction. This effect is called screening effect or shielding effect. The magnitude of screening effect depends upon the number of inner electrons. Higher the number of inner electrons, greater is the value of screening effect. The screening constant is represented by 'o' (sigma). Example : Across a period, the screening effect due to inner electrons remains the same as electrons are added to the same shell. Down the group, the screening effect due to inner electrons increases as a new valence shell is added. e.g. Potassium (19K) has electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1 K has 4 shells and thus, the valence shell electrons are effectively shielded by the electrons present in the inner three shells. As a result of this, valence shell electron (4s1) in K experiences a much less effective nuclear charge and can be easily removed.
(D) Why the second ionization enthalpy is greater than the first ionization enthalpy ?
First electron is removed from a gaseous neutral atom while second electron is removed from positively charged gaseous cation. Hence the second ionization enthalpy (IE2) is higher than the first ionization enthalpy (IE1). In general, if IE1, IE2 and IE3 are the first, second and third ionization enthalpies respectively then, IE3 > IE2 > IE1.
(E) Why the elements belonging to the same group do have similar chemical properties ?
The elements belonging to the same group have similar electronic configuration of the valence shell. These valence electrons are involved during a chemical reaction. Hence, they have similar chemical properties.
(F) Explain : electronegativity and electron gain enthalpy. Which of the two can be measured experimentally?
Question 4. Choose the correct option
(A) Consider the elements B, Al, Mg and K predict the correct order of metallic character :
(a) B > Al > Mg > K
(b) Al > Mg > B > K
(c) Mg > Al > K > B
(d) K > Mg > Al > B
(d) K > Mg > Al > B
(B) In modern periodic table, the period number indicates the :
(a) atomic number
(b) atomic mass
(c) principal quantum number
(d) azimuthal quantum number
(c) principal quantum number
(C) The lanthanides are placed in the periodic table at
(a) left hand side
(b) right hand side
(c) middle
(d) bottom
(d) bottom
(D) If the valence shell electronic configuration is ns2np5, the element will belong to
(a) alkali metals
(b) halogens
(c) alkaline earth metals
(d) actinides
(b) halogens
(E) In which group of elements of the modern periodic table are halogen placed ?
(a) 17
(b) 6
(c) 4
(d) 2
(a) 17
(F) Which of the atomic number represent the s-block elements ?
(a) 7, 15
(b) 3, 12
(c) 6, 14
(d) 9, 17
(b) 3, 12
(G) Which of the following pairs is NOT isoelectronic ?
(a) Na+ and Na
(b) Mg2+ and Ne
(c) Al3+ and B3+
(d) P3- and N3-
(b) Mg2+ and Ne
(H) Which of the following pair of elements has similar properties ?
(a) 13, 31
(b) 11, 20
(c) 12, 10
(d) 21, 33
(a) 13, 31
Question 5. Answer the following questions
(A) The electronic configuration of some elements are given below:
(a) 1s2 (b) 1s2 2s2 2p6
In which group and period of the periodic table they are placed ?
(a) 1s2 : Period first and group 18. (b) 1s2 2s2 2p6 : Period 2nd and group 18.
(B) For each of the following pairs, indicate which of the two species is of large size :
(a) Fe2+ or Fe3+
(b) Mg2+ or Ca2+
(a) Fe2+ (b) Ca2+
(C) Select the smaller ion form each of the following pairs:
(a) K+ , Li+ (b) N3-, F-
(a) Li+ (b) F-
(D) With the help of diagram answer the questions given below:
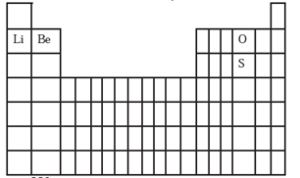
(a) Which atom should have smaller ionization enthalpy, oxygen or sulfur?
(b) The lithium forms +1 ions while berylium forms +2 ions ?
(a) Sulphur should have smaller ionization energy then oxygen. (b) Lithium (2, 1) has only one valence electron, hence forms +1 ion by the loss of 1 electron. Berylium (2, 2) has two valence electrons and form +2 ions by the loss of 2 electrons.
(E) Define :
(a) Ionic radius
Ionic radius is the distance between neighbouring cations and anions in ionic crystals.
(b) Electronegativity
The ability of a covalently bonded atom to attract the shared electrons towards itself is called electronegativity. (EN).
(F) Compare chemical properties of metals and non metals.
(G) What are the valence electrons ? For s-block and p-block elements show that number of valence electrons is equal to its group number.
Valence electrons are the electrons present in the outermost shell of an atom.

(H) Define ionization enthalpy. Name the factors on which ionisation enthalpy depends? How does it vary down the group and across a period?
Ionization enthalpy : The amount of energy required to remove an electron from the isolated gaseous atom in its ground state is called ionization enthalpy. X(g) → X(g)+ + e- ΔiH : [Ionization enthalpy is always positive as energy has to be supplied to remove outer electron from an atom.] Factors on which ionisation enthalpy depends are : (a) Radius of an atom (b) Nuclear charge (c) The screening effect of inner electrons (d) Nature of electronic configuration. Ionization enthalpy in the periods : Hence, along a period ionization enthalpy increases. In each period, the last inert element has the highest ionization enthalpy, and alkali metals have the lowest value of first ionization enthalpy. Ionization enthalpy in a group :
(I) How the atomic size vary in a group and across a period? Explain with suitable example.
(1) In a period : Atomic radius decreases in a period from left to right (upto group 17). This is because as we move across a period, atomic number increases from left to right, nuclear charge increases but electrons enter the same shell. Thus, the screening effect of the inner core remains the same, on the other hand the effective nuclear charge goes on increasing. As a result the valence electrons are tightly held by the nucleus and atomic size (radius) decreases. For example, in 2nd Period, it decreases from Li(152 pm) to F(64 pm). (2) In a group : Down the group, as the atomic number increase, the effective nuclear charge increases and shielding effect increases down a group. The valence electrons are held by weaker attrctive forces, thus, atomic radius increases. For example, atomic radius increases. For group 17 elements, the value increase from 1st member F (64 pm) to the last member At (140 pm).
(J) Give reasons.
(a) Alkali metals have low ionization enthalpies.
Alkali metals have large volume and only one electron in the outermost shell (ns1). Therefore, less energy is required to remove the single unpaired electron from the valence shell. Hence alkali metals have low ionization energy.
(b) Inert gases have exceptionally high ionization enthalpies.
Hence, inert gases have exceptionally high ionization enthalpies.
(c) Fluorine has less electron affinity than chlorine.
F(g) + e- → F- -328 kJ mol-1 Cl(g) + e- → Cl- -349 kJ mol-1
(d) Noble gases possess relatively large atomic size.
Noble gases (last element in each period) are inert elements, as their outermost shell is completely filled and the octet is completed. Thus, noble gases (group 18) have larger atomic radii than preceding halogens (group 17).
(K) Consider the oxides Li2O, CO2, B2O3.
(a) Which oxide would you expect to be the most basic?
(b) Which oxide would be the most acidic?
(c) Give the formula of an amphoteric oxide.
(a) Li2O is the most basic oxide. (b) CO2 would be the most acidic oxide B2O3 is an acidic oxide. (c) Al2O3 is an amphoteric oxide.
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6-Redox Reactions – Online Solutions
Next Chapter : Chapter-8-Elements of Groups 1 and 2 – Online Solutions
We reply to valid query.