Adsorption and Colloids
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -11
Solutions
Question 1. Choose the correct option.
(A) The size of colloidal particles lies between
(a) 10-10 m and 10-9 m
(b) 10-9 m and 10-6 m
(c) 10-6 m and 10-4 m
(d) 10-5 m and 10-2 m
(b) 10-9 m and 10-6 m
(B) Gum in water is an example of
(a) true solution
(b) suspension
(c) lyophilic sol
(d) lyophobic sol
(c) lyophilic sol
(C) In Haber process of production of ammonia K2O is used as
(a) catalyst
(b) inhibitor
(c) promotor
(d) adsorbate
(c) promotor
(D) Fruit Jam is an example of
(a) sol
(b) gel
(c) emulsion
(d) true solution
(b) gel
Question 2. Answer in one sentence :
(A) Name type of adsorption in which vander Waals forces are present.
Physical adsorption or physisorption involves van der Waals forces.
(B) Name type of adsorption in which compound is formed.
In chemical adsorption or chemisorption a compound is formed.
(C) Write an equation for Freundlich adsorption isotherm.
Freundlich adsorption isotherm is represented as : \(\frac{x}{m}=kp^{1/n}\) where : x = mass of adsorbate m = mass of adsorbent p = equilibrium pressure k and n = constants.
Question 3. Answer the following questions:
(A) Define the terms:
(a) Inhibition
Inhibition : The phenomenon of retarding a rate of a reaction in the presence of an inhibitor is called inhibition.
(b) Electrophoresis
Electrophoresis : The movement of colloidal particles under an applied electric potential is called electrophoresis.
(c) Catalysis.
Catalysis : The phenomenon of increasing the rate of a chemical reaction with the help of a catalyst is known as catalysis.
(B) Define adsorption. Why students can read black board written by chalks?
(a) Adsorption :The accumulation of a substance on the surface of another substance due to unsatisfied or unbalanced attractive forces on the surface is called adsorption. (b) When letters are written on black board by chalks, the particles of the chalks are adsorbed on the surface of the board. Hence students can read black board.
(C) Write characteristics of adsorption.
(D) Distinguish between Lyophobic and Lyophilic sols.
Lyophilic colloids
Lyophobic colloids
1. Formed easily by direct mixing.
Formed only by special methods.
2. Reversible.
Irreversible.
3. The particles are not easily visible even under ultramicroscope.
The particles are easily detected under ultramicroscope.
4. These are self stabilized.
These are unstable and hence require traces of stabilizers.
5. Addition of large amount of electrolytes causes precipitation /coagulation.
Addition of small amount of electrolytes causes precipitation/ coagulation.
6. Viscosity of dispelrsed phase much higher than that of the dispersion medium.
Viscosity of dispersed phase is nearly the same as the dispersion medium.
7. Surface tension of dispersed phase is lower than dispersion medium.
Surface tension of dispersed phase is nearly the same as the dispersion medium.
(E) Identify dispersed phase and dispersion medium in the following colloidal dispersions.
(a) milk (b) blood (c) printing ink (d) fog
Colloidal dispersion
Dispersed phase
Dispersion medium
milk
liquid
liquid
blood
solid
liquid
printing ink
solid
liquid
fog
liquid
gas
(F) Write notes on :
(a) Tyndall effect
Tyndall effect : “The phenomenon of scattering of light by colloidal particles and making path of light visible through the dispersion is referred as Tyndall effect.” Importance of Tyndall effect :

(b) Brownian motion
Brownian motion : The random ceaseless motion of the colloidal or disperse phase particles taking place in all directions over a largo area is called Brownian motion. Cause of Brownian motion :

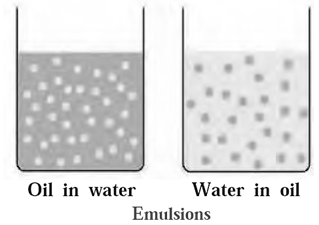
(c) Types of emulsion
There are two types of emulsions (i) Emulsion of oil in water (o/w type) : An emulsion in which dispersed phase is oil anddispersion medium is water is called emulsion of oil in water. For example : milk, vanishing cream, paint etc. Milk consists of particles of fat dispersed in water. (ii) Emulsion of water in oil (w/o type): An emulsion in which dispersed phase is water and dispersion medium is oil is called emulsion of water in oil. For example, codliver oil consists of particles of water dispersed in oil. Some other examples of this type include butter, cream, etc.
(d) Hardy-Schulze rule
Hardy-Schulze rule : The ability of the flocculating ion to precipitate is often inversely proportional to its valence. This is referred to as the Hardy-Schulze rule. (i) Ions of the added electrolyte neutralize oppositely charged colloidal particles in the colloidal solution and cause the precipitation. (ii) The precipitation power of an electrolyte increases with the increase in the valence of an anion or a cation of the electrolyte. For example, Na+ < Mg+ < Al2+ and Cl— < SO42— < PO43—
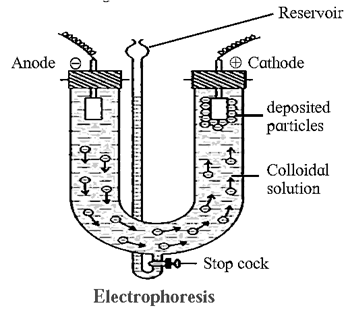
(G) Explain Electrophoresis in brief with the help of diagram. What are its applications ?
Electrophoresis : The movement of colloidal particles under an applied electric potential is called electrophoresis. Electrophoresis set up : Applications :
(H) Explain why finely divided substance is more effective as adsorbent?
Finely divided small size particles provide a large surface area compared to a large particle. Hence adsorption is to a greater extent. This increases the catalytic activity to a greater extent.
(I) What is the adsorption Isotherm?
Adsorption Isotherm : The relationship between the amount of a substance adsorbed per unit mass of an adsorbent and the equilibrium pressure (in case of gas) or concentration (in case of solution) at a given constant temperature is called an adsorption isotherm.
(J) Aqueous solution of raw sugar, when passed over beds of animal charcoal, becomes colourless. Explain.
Animal charcoal adsorbs the colouring matter in aqueous solution of raw sugar on its surface. Hence, the solution of sugar becomes colourless.
(K) What happens when a beam of light is passed through a colloidal sol?

(L) Mention factors affecting adsorption of gas on solids.
The adsorption of gases on solids depends on the following factors :
(M) Give four uses of adsorption.
(i) Catalysis: The solid catalysts are used in many industrial manufacturing processes. (ii) Gas masks: It is a device which consists of activated charcoal or mixture of adsorbents. It is used for breathing in coal mines to avoid inhaling of the poisonous gases. (iii) Control of humidity : Silica and alumina gels are good adsorbents of moisture. (iv) Production of high vacuum : Lowering of temperature at a given pressure, increases the rate of adsorption of gases on charcoal powder. By using this principle, high vacuum can be attained by adsorption.
(N) Explain Bredig's arc method.
Bredig’s Arc method :

(O) Explain the term emulsions and types of emulsions.
Emulsions : A colloidal system in which one liquid is dispersed in another immiscible liquid is called an emulsion. There are two types of emulsions (i) Emulsion of oil in water (o/w type) : An emulsion in which dispersed phase is oil anddispersion medium is water is called emulsion of oil in water. For example : milk, vanishing cream, paint etc. Milk consists of particles of fat dispersed in water. (ii) Emulsion of water in oil (w/o type): An emulsion in which dispersed phase is water and dispersion medium is oil is called emulsion of water in oil. For example, codliver oil consists of particles of water dispersed in oil. Some other examples of this type include butter, cream, etc.
Question 4. Explain the following :
(A) A finely divided substance is more effective as adsorbent.
Finely divided small size particles provide a large surface area compared to a large particle. Hence adsorption is to a greater extent. This increases the catalytic activity to a greater extent.
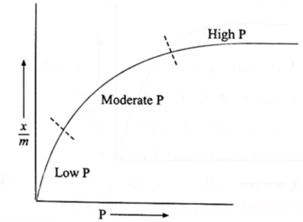
(B) Freundlich adsorption isotherm, with the help of a graph.
Graphical Representation of Freundlich equation : (1) When x/m is plotted against P, a smooth parabolic curve is obtained. (i) At low pressure : x/m increases linearly with the increase in pressure, Hence x/m ∝ P1. (ii) At high pressure : Adsorption is independent of P. Hence x/m ∝ P0. (m) At moderate pressure : x/m varies exponentially. x/m ∝ P1/n where 0 < 1/n < 1. (2) Taking the logarithm to the base 10 of both sides of Freundlich’s isotherm (equation), log10 \(\frac{x}{m}\) = \(\frac{1}{n}\) log10 C + log10 k This is an equation of a straight line (y = mx + c). If log10 \(\frac{x}{m}\) is plotted against log10 P, a straight line is obtained. The slope of this line is \(\frac{1}{n}\) and the intercept on log10 \(\frac{x}{m}\) axis is log10 k For a solution we can write, log10 \(\frac{x}{m}\) = \(\frac{1}{n}\)log10 C + log10 k

Question 5. Distinguish between the following :
(A) Adsorption and absorption. Give one example.
Adsorption
Absorption
Adsorption is a surface phenomenon as the adsorbed matter is concentrated only at the surface and does not penetrate through the surface to the bulk of adsorbent.
Absorption is a bulk phenomenon as the absorbed matter is uniformly distributed inside as well as at the surface of the bulk of a substance.
The concentration of the adsorbate is high only at the surface of the adsorbent.
The concentration of the absorbate is uniform throughout the bulk of the absorbent.
It takes place with the evolution of heat.
No heat is either evolved or absorbed.
It depends, upon temperature.
It is independent of temperature.
It depends upon the pressure of the adsorbate gas.
It is independent of pressure.
The rate of adsorption decreases with time since available surface area decreases.
It takes place with uniform rate.
Adsorption of H2(g) on Ni surface, adsorption of a gas or liquid-like acetic acid by activated charcoal.
Absorption of water by a sponge, absorption of water by cotton, absorption of ink by blotting paper.
(B) Physisorption and chemisorption. Give one example.
adsorbent particles. to 40 kJ mol-1 of an adsorbate. to 220 kJ mol-1 of an adsorbate.
Physisorption
Chemisorption
It involves physical or van der Waal's forces between adsorbate and adsorbent.
It involves formation of chemical or covalent bonds between adsorbate and
The heat of adsorption is low, about 20
The heat of adsorption is high about 40
It is relatively weak.
It is relatively strong.
It is non-specific.
It is specific.
It involves the formation of multilayers of adsorbate molecules on the surface of the adsorbent.
It involves the formation of monolayer of adsorbate molecules on the surface of the adsorbent.
It occurs at low temperature and the extent of adsorption decreases as the temperature of the surface increases.
It occurs at all temperature but the extent of adsorption increases with the rise in temperature.
The adsorbed adsorbate molecules desorb on heating, hence it is reversible.
Adsorbed adsorbate molecule don't desorb on heating hence it is irreversible.
It does not involve
It involves activation energy.
Adsorption of H2(g) on a catalyst.
Adsorption of O2(g) on a metal surface.
Question 6. Adsorption is surface phenomenon. Explain.
Question 7. Explain how the adsorption of gas on solid varies with
(a) nature of adsorbate and adsorbent
(b) surface area of adsorbent
(a) Nature of adsorbate: Nature of adsorbent: (b) Surface area of an adsorbent : Adsorption is a surface phenomenon, hence as the surface area increases, the extent of adsorption increases. For example, finely divided substances like charcoal powder, rough metal surfaces, colloidal substances, etc. are good adsorbents.
Question 8. Explain two applications of adsorption.
(i) Separation of inert gases : In a mixture of noble gases, different gases adsorb to different extent. Due to selective adsorption principle, gases can be separated on coconut charcoal. (ii) Froth floatation process : A low grade sulfide ore is concentrated by separating it from silica and other earthy matter using pine oil as frothing agent. Hydrophobic pine oil preferentially wets (adsorbs on) sulfide ore which is taken up in the froth.
Question 9. Explain micelle formation in soap solution.
Micelle formation in a soap solution :

Question 10. Draw labelled diagrams of the following :
(a) Tyndall effect

(b) Dialysis

(c) Bredig's arc method

(d) Soap micelle

Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-10-States of Matter – Online Solutions
Next Chapter : Chapter-12-Chemical Equilibrium – Online Solutions