Chapter-5-Chemical Bonding
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -5
Notes Part-2
Topics to be Learn : Part-2
|
Advanced theories of Bonding :
Two important approaches regarding nature of chemical bond are valence bond theory and molecular orbital theory
Valence bond theory :
Heitler and London developed the valence bond theory in 1926 on the basis of wave mechanics. This theory was further extended by Pauling and Slater in 1931.
Postulates of Valence Bond Theory :
- A covalent bond is formed only when half filled orbitals of two atoms overlap each other. In the process, energy of the system decreases.
- Each overlapping atomic orbital should contain an unpaired electron with opposite spin.
- On overlapping of orbitals, electron spins are neutralised and electrons get paired up.
- The overlapping atomic orbitals must have comparable energies (nearly same energy).
- During the overlap of atomic orbitals, electron density increases between two nuclei and therefore repulsion between the two nuclei of bonded atoms decreases. This results in liberation of energy and increase in the attractive forces between the atoms.
- A bond is formed at an equilibrium distance when the system has minimum potential energy and maximum stability.
- The strength of a covalent bond depends upon the extent of overlap between the two atoms. Greater the extent of overlap, more is the energy released and stronger is the bond formed.
- The number of covalent bonds formed by an atom of element is equal to the number of unpaired electrons present in the valence shell of the atom.
- Covalent bond formed by overlap of atomic orbitals has directional characteristics as each atomic orbital except s-orbital has a particular direction in space.
- Geometry of a molecule is decided by directional orientation of overlapping atomic orbitals.
Interacting forces during covalent bond formation :
The interacting forces and energy changes that take place during the formation of a covalent bond explained below.
When two combining atoms approach each other during the formation of a covalent bond, the following forces come into play.
- Forces of repulsion : (a) The nucleus of one atom repels the nucleus of other atom and vice versa. (b) The electron of one atom repels the electron of the other atom and vice versa.
- Forces of attraction : The nucleus of one atom attracts the electron of the other atom and vice versa.
When the magnitude of the attractive forces becomes more than that of repulsive forces, the energy of the system decreases and a covalent bond is formed. Greater the decrease in energy, more is the stability and stronger is the covalent bond formed.
When the magnitude of repulsive forces becomes more than that of attraction, the total energy of the system increases and a covalent bond is not formed.
When both the forces become equal, the energy of the system becomes minimum and a stable bond is formed between two atoms.
Potential energy diagram for the formation of H2 molecule :
The lowering of energy during bond formation is depicted in the potential energy diagram. To understand this let us consider the formation of H2 molecule from atoms of hydrogen each containing one unpaired electrons.
- Hydrogen molecule has two H—atoms, having electronic configuration, 1H = 1s1.
- When the two bonding atoms Ha and Hb having electrons with opposite spins are far apart from each other at a distance ‘A’, there is no interaction between them. The total potential energy is assumed to be zero.
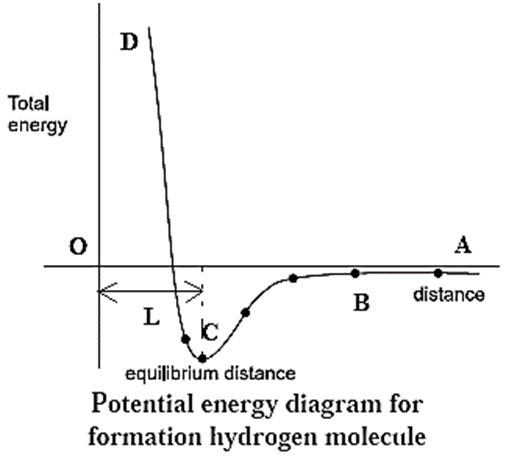
- As the two atoms with opposite spins, approach each other, after a certain distance B, the interaction begins and the following forces begin to operate on them :
- Attractive forces between : electron of Ha and nucleus of Hb and electron of Hb and nucleus of Ha.
- Repulsive forces between : electron of Ha and electron of Hb and nucleus of Ha and nucleus of Hb.
- In the beginning, attractive forces are greater than repulsive forces and the potential energy of the system decreases as internuclear distance decreases.
- Ultimately an equilibrium stage is reached at C when attractive forces are balanced by repulsive forces and potential energy is at a minimum value.
- At this stage, two hydrogen atoms are bonded together to form a stable molecule H2. The internuclear distance is L (74 pm) which is the H—H bond length.
- The energy released is called bond enthalpy (435-8 kJ mol-1 -corresponding to minimum in the potential energy curve).
- If the internuclear distance is further decreased after the point C, the repulsive forces become greater than the attractive forces and the potential energy increases along CD. Hence ABCD is a potential energy curve for the formation of H2 molecule.
- If the hydrogen atoms Ha and Hb have identical parallel spins, the potential energy of the system increases continuously as they approach each other and the bond formation does not take place.
Overlap of atomic orbitals :
Formation of a bond has been explained on the basis of overlap of atomic orbital having same energy and symmetry.
The orbitals holding the electrons vary in shape, energy and symmetry. The extent of overlap depends on the shape and size of the orbital
On the basis of the above considerations there are 2 types of covalent bonds.
- sigma bond (σ)
- pi bond (π)
- Sigma (σ) bond : The covalent bond formed by the linear or coaxial overlap of two half filledatomic orbitals of two bonding atoms along the internuclear axis, is called a sigma (σ) bond. The sigma (σ) bond can be formed by s—s, s-p or coaxial p-p overlapping of atomic orbitals.
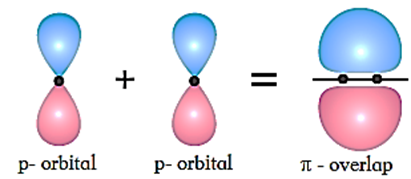
- Pi (π) bond: The covalent bond formed by lateral or sidewise overlap of two half filled symmetric atomic orbitals (like p-orbitals) of two atoms is called a pi (π) bond.
The σ bond is formed by the overlap of following orbitals.
- Two 's' orbitals
- One 's' and one pz orbital
- Two 'p' orbitals
s-s overlap :
e.g. H2 Or The formation of hydrogen molecule on the basis of VBT :
The overlap between two half filled s—orbitals of two different atoms having unpaired electrons with opposite spins is called s-s overlap.
Example : Formation of H2 molecule.
- The electronic configuration of hydrogen atom is 1H = 1s1.
- During the formation of H2 molecule, half filled 1s—orbital of one hydrogen atom containing unpaired electron overlaps axially with half filled 1s-orbital of another hydrogen atom having unpaired electron with opposite spin.
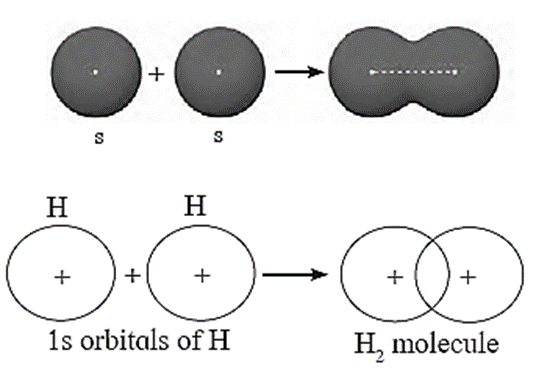
- Thus, a molecule of H2 is formed by s—s overlap.
- The bond formed is s-s sigma (σ) bond which is a strong bond due to maximum overlap of orbitals.
p-p overlap :
The overlap between two half filled p-orbitals of two atoms containing electrons with opposite spin is called p-p overlap. p-p overlap may be axial or lateral.
Example : Formation of fluorine (F2) molecule.
- Electronic configuration of fluorine atom is 9F = 1s2, 2s2 2px2, 2py2 2pz1.
- During the formation of F2 molecule, half filled 2pZ-orbital of one F-atom overlaps axially with half filled 2pZ -orbital of another F atom having electron with opposite spin.
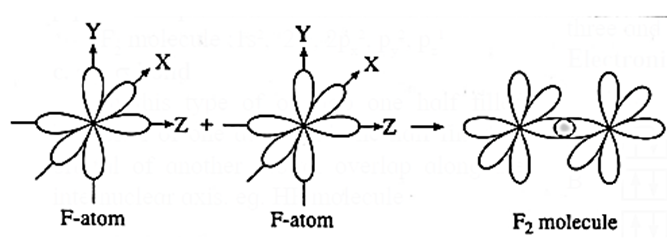
- Thus, F2 molecule is formed as a result of p-p overlap.
- The bond formed is a p—p σ bond :
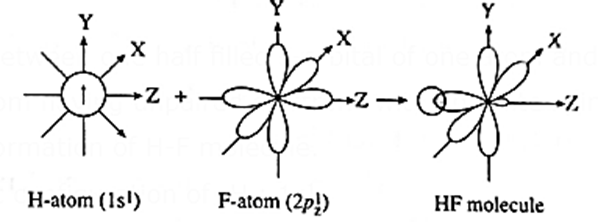
s-p σ bond :
The overlap between one half filled s-orbital of one atom and one half filled p-orbital of another atom having unpaired electrons with opposite spin is called s-p overlap.
Example : Formation of H-F molecule.
The electronic configuration of 1H : 1s1
9F ; 1s2, 2s2 2px2, 2py2 2pz1.
- During the formation of H-F molecule, half filled 1s-orbital of hydrogen atom overlaps axially with half filled 2pz-orbital of fluorine atom having unpaired electron with opposite spin.
- Thus, a molecule of H—F is formed as a result of s—p overlap.
- The bond formed is s—p σ bond which is a strong bond since the axial overlap is maximum.
p-p overlap/π overlap/π bond :
When two half filled orbitals of two atoms overlap side ways (laterally) it is called π overlap and it is perpendicular to the internuclear axis.
Hybridization : The process of mixing and recasting of atomic orbitals of the same atom with slightly different energies to form equal number of new orbitals with equivalent energy, maximum symmetry and definite orientation in space is called hybridisation and the orbitals formed are called hybrid or hybridized orbitals.
Need for the concept of hybridization :
The concept of hybridisation was introduced by Pauling and Slater as a result of failure of valence bond theory to explain the following points.
(i) Valencies of certain elements : Valency of an element is equal to the number of unpaired electrons in the atoms. However, valency of certain elements cannot be explained on this basis.
For example :
- Beryllium has electronic configuration 1s2, 2s2. The expected valency is zero (as there is no unpaired electron) but the observed valency is 2 as in BeCl2.
- Boron has electronic configuration 1s2, 2s2 2px1. The valency should be 1 but it is 3 as in BF3.
- Carbon has electronic configuration 1s2, 2s2 2px1, 2py1 The valency should be 2 but observed valency is 4 as in CH4.
(ii) The shapes and geometry of certain molecules : The valence bond theory cannot explain shapes, geometry and bond angles in certain molecules.
For example :
- Tetrahedral shape of methane molecule.
- Bond angles in molecules like NH3 (107°18') and H2O (104°35’) molecules.
But all these points can be explained only by the concept of hybridisation, which explains its need.
Hybridization refers to mixing of valence orbitals of same atom and recasting them into equal number of new equivalent orbitals-Hybrid orbitals.
Steps considered in Hybridization :
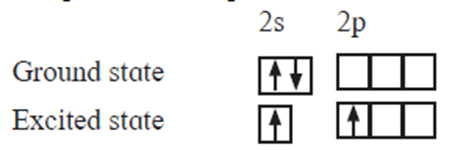
Formation of an excited state : When the number of unpaired electrons present in the ground state of an atom of an element is not equal to (or less than) the valency of the element, an atom (in the ground state) absorbs energy and promotes one or more electrons from filled orbitals of lower energy to the vacant orbitals of higher energy, forming an excited state. In this state, number of unpaired electrons is equal to the valency of the atom.
e.g. in BeF2, valency of Be is two. In the excited state one electron from 2s orbital is uncoupled and promoted to 2p orbital.
Mixing and recasting of atomic orbitals : Depending upon the requirement of bonding, some or all the orbitals of the valence shell of the atom mix and reform a new set of equivalent orbitals having the same energy. These new orbitals formed are called hybridized orbitals. The number of hybridised orbitals is equal to the number of hybridising orbitals.
Orientation of hybrid orbitals in space : The hybrid orbitals orient themselves in space so as to minimize inter—electron repulsion to acquire stability(minimum repulsion and maximum separation between them). This arrangement gives a specific geometry to the molecule.
So during formation of sp hybrid orbitals as in Be the two sp hybrid orbitals are 1800.
Conditions for hybridisation :
- Atomic orbitals of the same atom undergo hybridisation.
- Hybridising atomic orbitals should have nearly the same energy, so 2s and 2p orbitals undergo hybridization but 3s and 2p orbitals do not.
- Only atomic orbitals and not electrons undergo hybridisation.
Characteristic features of hybrid orbitals :
- Number of hybrid orbitals formed is exactly the same as the participating atomic orbitals.
- They have same energy and shape.
- Hybrid orbitals are oriented in space in such a way that there is minimum repulsion and thus are directional in nature.
- The hybrid orbitals are different in shape from the participating atomic orbitals, but they bear the characteristics of the atomic orbitals from which they are derived.
- Each hybrid orbitals can hold two electrons with opposite spins.
- A hybrid orbital has two lobes on the two sides of the nucleus. One lobe is large and the other small.
- Covalent bonds formed by hybrid orbitals are stronger than those formed by pure orbitals, because the hybrid orbital has electron density concentrated on the side with a larger lobe and the other is small allowing greater overlap of the orbitals.
Types of Hybridization and Geometry of Molecules : Different types of hybrid orbitals are obtained from the atomic orbitals that participate in hybridization. s and p orbitals can hybridize to form the following hybrid orbitals
- sp3
- sp2
- sp
sp3 Hybridization : In this type one 's' and three 'p' orbitals having comparable energy mix and recast to form four sp3 hybrid orbitals.
- In this type 's' orbital is spherically symmetrical while the px, py, pz, orbitals have two lobes and are directed along x, y and z axes, respectively.
- The four sp3 hybrid orbitals formed are equivalent in energy and shape.
- They have one large lobe and one small lobe.
- They are at an angle of 109028’ with each other in space and point towards the corners of a tetrahedron
- CH4, NH3, H2O are examples where the orbitals on central atom undergo sp3 hybridization.
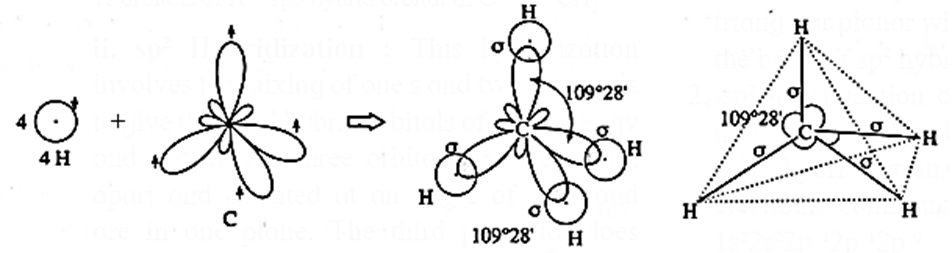
Geometry of methane molecule on the basis of sp3-hybridisation :
(i) Methane (CH4) molecule has one carbon atom and four hydrogen atoms.
(ii) Electronic configuration of H (= 1) : 1s1
C (=6) : 1s2, 2s2, 2px1, 2py1.
(iii) The central carbon atom undergoes sp3-hybridisation. Carbon is 1s1, 2s2, 2px1, 2py1 2pz0. In order to form four equivalent bonds with hydrogen the 2s and 2p orbitals undergo hybridization.
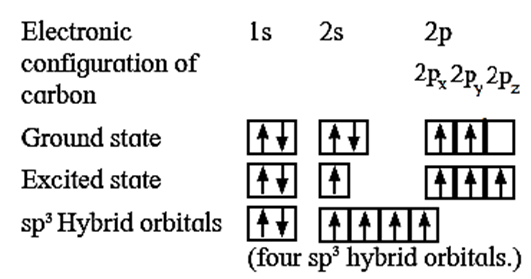
(iv) Tetravalency of carbon : In the ground state, carbon atom has only two unpaired electrons, hence, it should be divalent. But its tetravalency is explained by excitation of an electron from lower energy 2s—orbital to the higher energy vacant 2pz-orbital giving four unpaired electrons.
(v) sp3-Hybridisation : In this, one s-orbital and three p-orbitals of C atom undergo sp3-hybridisation forming four equivalent sp3-hybridised orbitals directed towards four corners of tetrahedron with the angle 109°28’.
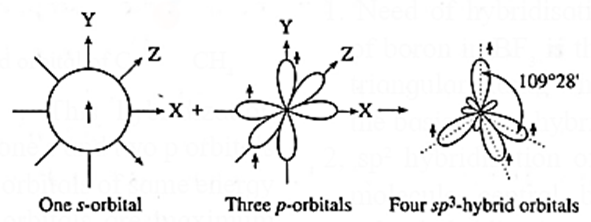
(vi) Formation of bonds : Each sp3-hybridised orbital of carbon atom overlaps coaxially with 1s-orbital of each hydrogen atom having unpaired electron with opposite spin forming four sp3-s sigma (σ) bonds.
- Therefore the geometry of methane molecule is tetrahedral.
- Each H—C—H bond angle is 109°28’.
Geometry of ammonia (NH3) molecule on the basis of hybridization :
(i) Ammonia (NH3) molecule has one nitrogen atom and three hydrogen atoms.
(ii) Electronic configuration of H(= 1) 1s1
N(= 7) 1s2, 2s2, 2px1, 2py1 2pz1.
(iii) The central nitrogen atom undergoes sp3-hybridisation.
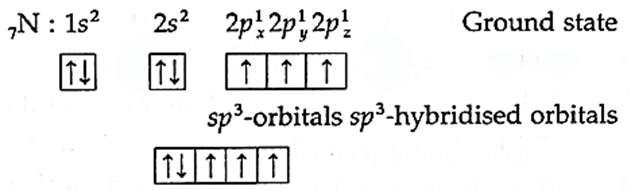
(iv) Need of hybridisation : In the ground state, nitrogen atom has three unpaired electrons in p-orbitals which can form three covalent bonds with three hydrogen atoms. As these p-orbitals are at 90°, the expected H—N—H bond angle is 90°.
However, the actual bond angle is 107°18’ which can be explained on the basis of sp3-hybridisation.
(v) sp3-hybridisation: In this, one s-orbital and three p-orbitals of N atom undergo sp3—hybridization forming four sp3-hybridised orbitals directed towards the four corners of regular tetrahedron. One of the sp3-hybrid orbitals has a lone pair of electrons.
(vi) Formation of bonds :Three sp3—hybridized orbitals overlap coaxially with 1s—orbital of each hydrogen atom having unpaired electron with opposite spin forming three sp3-s sigma (σ) bonds.
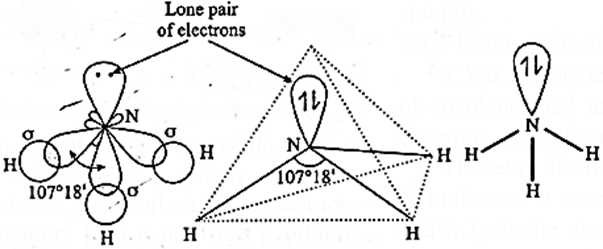
(vii) Geometry and bond angle in ammonia molecule: In ammonia molecule, one of the sp3—orbitals has a lone pair of electrons, due to which, there is a repulsion between lone—pair and bonding pair of electrons.
- As a result, the H—N—H bond angle is reduced from regular tetrahedral angle 109028’ (Lone pair-bond pair > bond pair-bond pair) to 107°18’.
- The geometry of the ammonia molecule is distorted tetrahedral or pyramidal.
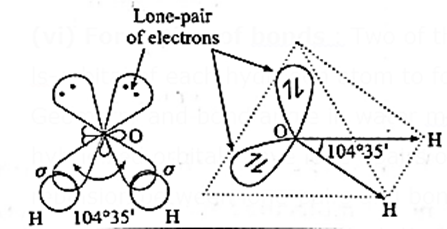
Geometry of water (H2O) molecule on the basis of hybridization :
(i) Water (H2O) molecule has one oxygen atom and two hydrogen atoms.
(ii) Electronic configuration : H(= 1) 1s1
O(= 8) 1s2, 2s2, 2px2, 2py1 2pz1.
(iii) The central oxygen atom undergoes sp3—hybridisation :
(iv) Need for hybridisation: In the ground state, oxygen atom has two unpaired electrons in p—orbitals which can form two covalent bonds with two atoms of hydrogen. However, as these p—orbitals are at 90° with each other, the expected H—O~H bond angle is 90°. But the observed bond angle is 104°35’ which can be explained on the basis of hybridisation.
(v) sp3-hybridisation: In this, one s—orbital and three p-orbitals of oxygen atom undergo sp3-hybridisation forming four sp3-hybridised orbitals directed towards four corners of a tetrahedron. Two of the hybrid orbitals have lone-pair of electrons.
(vi) Formation of bonds : Two of the sp3-hybridised orbitals overlap coaxially with ls-orbital of each hydrogen atom to form two sp3-s sigma (σ) covalent bonds.
Geometry and bond angle in water molecule : In water molecule, two of the sp3—hybridized orbitals have lone—pairs of electrons due to which there is force of repulsion between lone-pairs and bonding pairs.
- These forces cause distortation in the H—O—H bond angle which is reduced from the expected regular tetrahedral angle 109°28’ to 104°35’.
Hence the geometry of water molecule is angular or ’V’shaped
sp2-hybridisation : This hybridisation involves the mixing of one s—orbital and two p—orbitals to give three sp2—hybrid orbitals of equivalent energy and shape.
These three hybrid orbitals are maximum apart and oriented at an angle of 120° and are in one plane. For example, BF3, C2H4 molecules.
Geometry of boron trifluoride (BF3) molecule on the basis of hybridization :
(i) Boron trifluoride (BF3) molecule has one boron atom and three fluorine atoms.
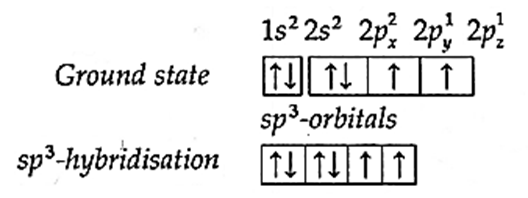
(ii) In BF3 molecule central boron atom undergoes sp2 hybridisation. The ground state electronic configuration of Boron (z = 5) is 1s2, 2s2, 2px1, 2py0 2pz0.
(iii) One of the paired electrons from 2s-orbital is excited to empty 2py-orbital giving three unpaired electrons for trivalency of B. The central boron atom undergoes sp2-hybridisation.
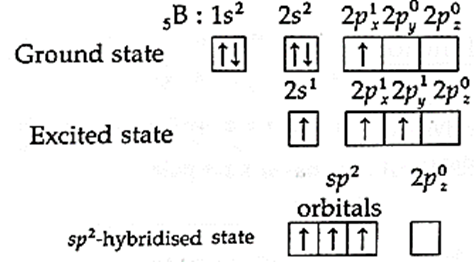
(iv) sp2 –hybridisation :In this, one s—orbital and two p-orbitals of boron atom undergo sp2-hybridisation forming three sp2-hybridised orbitals directed towards the three corners of an equilateral triangle with angle of 120°.
(v) Formation of bonds: Each sp2-hybridised orbital overlaps coaxially with 2pz—orbital of fluorine atom forming three sp2-p sigma (σ) bonds.
- Geometry of the molecule is trigonal planar.
- The F—B—F bond angle is 120°.

Formation of ethene (ethylene) molecule on the basis of hybridization :
(i) Ethene (C2H4) molecule has two carbon atoms and four hydrogen atoms.
(ii) Electronic configuration : H(= 1) 1s1
C(=6) 1s2, 2s2, 2px1, 2py1
(iii) One of the paired electrons from 2s—orbital is excited to the vacant 2pz orbital giving four unpaired electrons for tetravalency of C atom. In this, each carbon atom undergoes sp2—hybridisation.
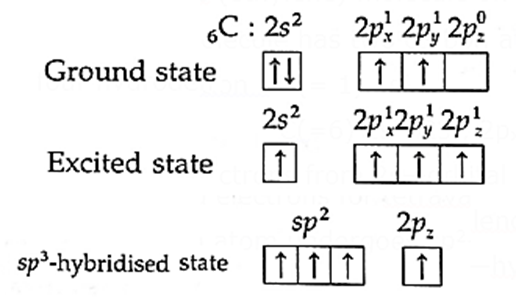
(iv) Need for hybridisation : The observed H—C—H bond angle and formation of TC bond between two carbon atoms can be explained by sp2-hybridisation.
(v) sp2—hybridisation: In this, one s—orbital and two p-orbitals of carbon atom undergo sp2-hybridisation forming three sp2-hybridised orbitals directed towards the three corners of an equilateral triangle with the angle of 120°. 2pz-orbital remains unhybridised.
Formation of bonds :
- Each sp2-hydridised orbital of each carbon atom overlaps coaxially with 1s-orbitals of two hydrogen atoms forming two C-H (sp2—s) sigma (σ) bonds.
- The remaining sp2-hybriclised orbital of one carbon atom overlaps axially with sp2-hybridised orbital of another carbon atom to form a C—C (sp2—sp2) sigma (σ) bond.
- The unhybridised half filled 2p2—orbitals of two carbon atoms having electrons with opposite spins overlap laterally to form aC—C (2p2—2p2) pi (π) bond.
- Thus, in ethene molecule, there are four C—H sigma bonds, one C—C sigma bond and one C—C pi bond.

- Geometry of ethene molecule is trigonal planar
- All four C—H bonds are coplanar.
- The H—C—H bond angle is 120°.
sp-hybridisation :
This hybridisation involves the mixing of one s-and one p-orbitals to give two sp—hybridized orbitals of equivalent energy and shape arranged at 180° to each other.
For example, BeF2 and C2H2.
Geometry of beryllium difluoride (BeF2) molecule on the basis of hybridization :
(i) Beryllium difluoride (BeF2) has one beryllium atom and two fluorine atoms.
(ii) Electronic configuration : Be(= 4) : 1s2, 2s2, F(= 9) 1s2, 2s2, 2px2, 2py2, 2pz1
(iii) The central beryllium atom undergoes sp-hybridisation.
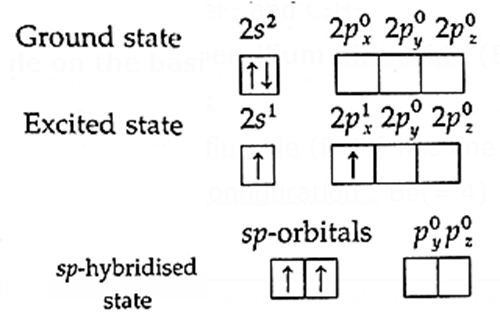
(iii) Divalency of beryllium atom : In the ground state beryllium atom has no unpaired electron, hence, the valency of Be should be zero. The divalency of beryllium atom is explained by excitation of one of the paired electrons from lower energy 2s-orbital to the higher energy vacant 2px-orbital giving two unpaired electrons.
(iv) sp-hybridisation : In this, one s-orbital and one p-orbital of Be undergo sp-hybridisation forming two sp-hybridised orbitals which lie along the same axis on opposite sides with the angle 180°.
(v) Formation of bonds :Two sp—hybridized orbitals of beryllium atom overlap axially with half filled 2p-orbitals of two fluorine atoms forming two Be-F (sp-p) sigma bonds.
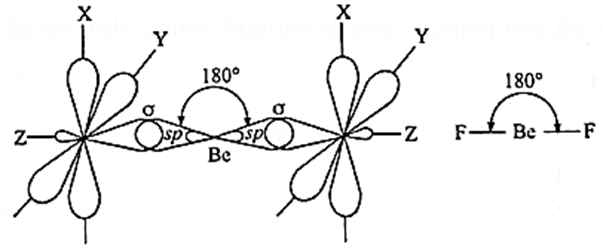
- Geometry of BeF2 molecule is linear or diagonal with F-Be-F bond angle 180°.
Geometry of ethyne molecule on the basis of hybridization:
(i) Ethyne (C2H2) molecule has two carbon atoms and two hydrogen atoms.
(ii) Electronic configuration : H(= 1) 1s1
C(=6) 1s2, 2s2, 2px1, 2py1
(iii) One of the paired electrons from 2s—orbital is excited to empty 2pz-orbital giving four unpaired electrons for tetravalency of C atom. Each carbon atom undergoes sp—hybridisation.
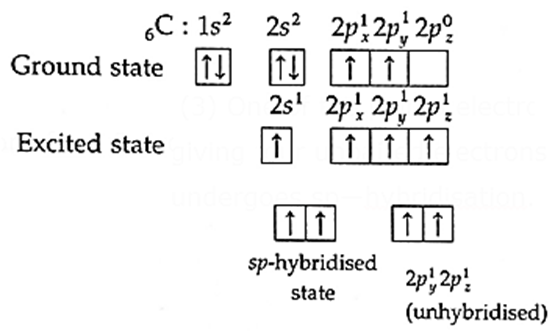
(iv) One s-orbital and one p—orbital of C atom undergo hybridisation forming two sp—hybridized orbitals which lie along a straight line in opposite direction with angle 180°. Two orbitals namely 2py and 2pz of each carbon atom remain unhybridised.
(v) Formation of bonds :
- One of the two sp-hybridised orbitals of two carbon atoms overlap axially with similar sp-hybrid orbital of another carbon atom to form C—C (sp—sp) sigma (σ) bond.
- The remaining sp-hybridised orbitals of two carbon atoms overlap axially with 1s-orbital of hydrogen atom forming two C—H (sp—s) sigma (σ) covalent bonds.
- The unhybridised 2py and 2pz-orbitals of each carbon atom overlap laterally respectively to form two C—C pi (π) bonds.
- Thus, in ethyne molecule, there are two C—H sigma bonds, one C—C sigma bond and two C—C pi bonds.
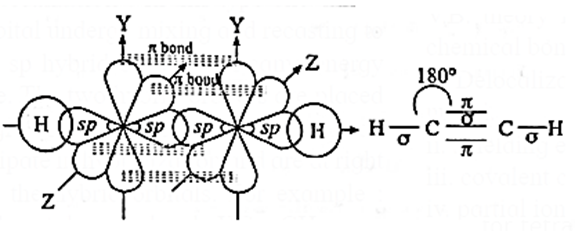
- Hybridisation of ethyne molecule is sp-hybridisation.
- Geometry of ethyne molecule is linear.
- The bond angle is 180°.
Importance and limitation of valence bond theory :
Importance of valence bond theory (V.B) :
V.B. theory introduced five new concepts in chemical bonding as follows :
- Delocalization of electron over the two nuclei.
- shielding effect of electrons.
- covalent character of bond.
- partial ionic character of a covalent bond.
- The concept of resonance and connection between resonance energy and molecular stability.
Limitations of valence bond theory :
- Valence bond theory explains the formation of a covalent bond formed by sharing of a pair of electrons between two bonding atoms. But does not explain the formation of a co-ordinate covalent bond.
- The paramagnetic character of O2 molecule cannot be explained.
- It does not explain the bonding and stability of electron deficient molecules like B2H6.
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-4-Structure of Atom – Online Notes
Next Chapter : Chapter-6-Redox Reactions – Online Notes
We reply to valid query.