Chemistry in Everyday Life
Maharashtra State Board-Class-11-Science-Chemistry-Chapter -16
Notes
|
Topics to be Learn
|
Introduction :
Chemistry is crucial in various life processes and has been utilized in human civilization for various purposes. Modern science has advanced synthetic organic chemistry, revolutionizing materials used in basic needs like food, clothing, and shelter, influencing various aspects of human life.
Basics of food chemistry :
Nutrients : Food provides essential nutrients that the body uses as energy sources, regulate growth, maintain and repair body tissues.
- The nutrients consist of carbohydrates, lipids, proteins, vitamins, minerals and water.
- Grains, fruits and vegetables provide carbohydrates and vitamins.
- Meat, fish, eggs, dairy products and pulses provide proteins and vitamins.
- Lipids are provided by vegtetable oils, dairy products and animal fats.
Food digestion process : Food digestion involves the breakdown of polymeric proteins and carbohydrates into monomers like α- amino acids and glucose, facilitated by enzymes.
Effect of cooking : During the cooking process, high polymers of carbohydrates or proteins are hydrolysed to smaller polymers. Due to the smaller size of the resulting constituent nutrient molecules, cooking makes food easy to digest.
Food quality chemistry :
Food preservation and processing methods aim to prevent undesirable changes and enhance desirable ones.
The following cases illustrate some aspects of food quality and the underlying chemisty :
(i) Browning of cut fruit/vegetables :
- Cutting action damage the cells resulting in release of polyphenols (chemicals).
- The polyphenols released are oxidised with oxygen in the presence of enzyme to form quinones.
- Quinone undergo polymerisation giving brown coloured products named tannins.
- Therefore, on cutting some fruits and vegetables they turn brown.
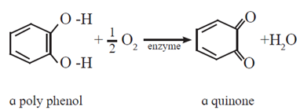
This browning reaction can be slowed down using reducing agents such as SO2, ascorbic acid (vitamin C) or by change of pH by adding edible acid such as lemon juice (citric acid) or vinegar.
- Example : Browning of cut apple is due to the coating of tannin. This browning process can be prolonged using reducing agent such as ascorbic acid (vitamin C) or by change of pH by adding edible acid such as lemon juice (citric acid).
(ii) Rancidity of oils and fats :
- On keeping for long time, oils and fats develop an unpleasant or rancid smell and disagreeable taste.
- The cause of rancidity is release of fatty acid produced during hydrolysis of fats brought about by water present in food. The hydrolysis of fats occur rapidly in the presence of certain microorganisms. It is an enzyme catalysed reaction.
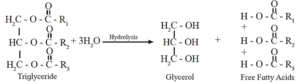
- The other cause of rancidity of oils and fats is oxidation by molecular oxygen in the air. The mono or polyunsaturated fat molecules break-down during the oxidation and form volatile aldehydes and carboxylic acids which give the unpleasant rancid taste. This is called oxidative rancidity.
Examples :
- The rancidity of milk and butter is due to the release of four, six and eight carbon fatty acids (butanoic, hexanoic and octanoic acids) on hydrolysis.
- Chocolate develops oily or fatty flavour due to release of palmitic, stearic and oleic acids on hydrolysis.
- Lauric acid on hydrolysis gives a soapy flavour to coconut oil.
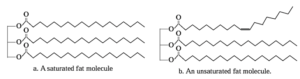
(iii) Saturated, unsaturated and trans fats :
Saturated Fats :
- Animal fats contain saturated fatty acids.
- Long chains of tetrahedral carbon atoms in a saturated fatty acid gets packed closely together.
- Also, van der Waals forces between the long chains are sufficiently strong to saturated fats into solid form at room temperature.
- Examples : Butter fat, vanaspati ghee, margarine saturated fats.
Unsaturated Fats :
- The long carbon chains of unsaturated fatty acids contain one or more C = C double bond.
- This produces one or more kinks in the chain, which prevents the molecules from packing closely together. The van der Waals forces between the unsaturated chains are weak. Therefore, the melting points of unsaturated fats are low.
Examples :
- Mono-unsaturated fats : Olive oil, peanut oil, canola oil.
- Poly-unsaturated fats : Sunflower oil, soyabean oil, fish oil.
Trans fats :
Unsaturated Fatty Acids: Cis and Trans Isomers
- Cis form: Two hydrogens on double-bonded carbons on same side.
- Trans form: Two hydrogens on opposite sides.
- Cis isomer is common in unsaturated fats.
- Trans form found only in animal and processed fats.
- Trans fats are difficult to metabolize and can accumulate dangerous levels in fatty tissue.
Lipoprotein : Saturated or trans unsaturated fats in the form of lipoprotein are used in the body for transport of cholesterol. Excessive LDL (low density lipoprotein) results in deposition of cholesterol in blood vessels which increases risk of cardio vascular disease.
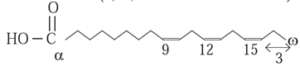
(iv) Omega-3 :
- Omega-3 fatty acids are present in certain natural, unsaturated fats.
- They name Omega—3 is given for the position of the double bond in a long carbon chain of the fatty acid.
- Omega denotes the last carbon of the carbon chain.
- Omega—3 fatty acids have C=C bond between the third and fourth carbon from the end of a carbon chain.
- For example, Linolenic Acid (9, 12, 15 —Octadecatrienoic acid)
- Omega—3 fats raise the high density protein HDL (good cholesterol) level of blood, and the beneficial for our health.
- Foods such as walnuts, flaxseeds, chia seeds, soyabean, cod liver oil are rich in Omega—3 fatty acids.
(v) Antioxidants as food additives :
An antioxidant is a substance that delays the onset of oxidation or slows down the rate of oxidation of food stuff.
It is used to extend the shelf life of food.
Antioxidants react with oxygen-containing free radicals and thereby prevent oxidative rancidity.
Example :
Vitamin E (tocopherol) : It is added to packed edible oils since it is very effective oxidant. The phenolic OH group in its structure is responsible for its antioxidant activity, while the long chain of saturated carbon makes it fat soluble.
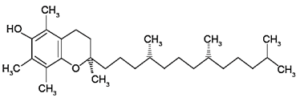
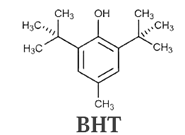
Synthetic antioxidants :
- Common structural units found in synthetic antioxidants are phenolic OH group and tertiary butyl group.
- For economic reason synthetic antioxidants are used as additives to increase the shelf-life of packed foods.
- For example, BHT, which is 3, 5-di-tertbutyl-4-hydroxytoluene.
Compounds with medicinal properties :
Drug : A chemical which interacts with biomolecules such as carbohydrates, lipids, proteins and nucleic acid and produces a biological response is called drug.
- Drugs being foreign substances in a body, often give rise to undersirable, adverse side effects.
Medicines : A drug having therapeutic and useful biological response is used as medicine. Medicines are used in diagnosis prevention and treatment of a disease.
Drug design : It is an important branch of medicinal chemistry which aims at synthesis of new molecules having better biological response.
Analgesics and antipyretics
Analgesics : Drugs which give relief from pain are called analgesics.
Example : Aspirin (Acetyl salicylic acid - C9H8O4 ), Paracetamol.
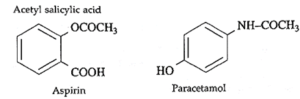
Antipyretics : Drugs which are used to reduce fever are called antipyretics.
Antimicrobials :
(i) Antiseptics and disinfectants :
Antiseptics : Drugs which are applied to the living tissues to kill the bacteria and to stop their growth, thus preventing its infection are called antiseptics.
- Antiseptics are used to apply on wound, cuts, ulcers, diseased skin surfaces.
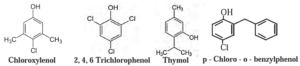
Examples :
- Iodine is a powerful antiseptic. Its 2-3 per cent solution in alcohol-water is known as tincture of iodine. Iodoforrn is also used as an antiseptic for wounds.
- A dilute aqueous solution of boric acid is a weak antiseptic used for eyes.
- Thymol obtained from oil of thyme is an excellent non toxic antiseptic.
- The popular dettol contains most common phenol derivatives like a diluted solution (4.8% w/v) chloroxylenol and terpineol. It serves as a powerful antiseptic.
Disinfectants : Disinfectants are non-selective antimicrobials, which kill a wide range of microorganisms including bacteria.
- Disinfectants are used on non-living surfaces like floors, instruments, sanitary ware.
- Examples : Concentrated solution of phenol, sulphur dioxide, chlorine, etc.
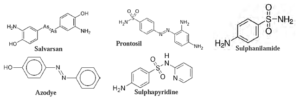
Name and Structure of some Antiseptics and disinfectants :
(ii) Antibiotic : It is a drug derived from microorganisms (bacteria, fungi or molds) or purely synthetic, used to kill or prevent the growth of other microorganisms.
- Examples : Penicillin, chloramphenicol, salvarsan, etc.
Antibiotics can be of three types. They are synthetic, semisynthetic or of microbial origin.
- broad spectrum (effective against wide range of bacteria),
- narrow spectrum (effective against one group of bacteria)
- limited spectrum (effective against single organism).
(a) Broad spectrum antibiotics : Those antibiotics which are effective against a wide range of gram positive and gram negative bacteria are known as broad spectrum antibiotics.
Examples : Chloramphenicol, ampicillin, amoxicillin. Broad spectrum antibiotics also kill the useful bacteria in the alimentary canal, that is their major disadvantage.
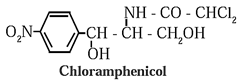
(b) Narrow spectrum antibiotics : Those antibiotics which are effective against either gram positive or gram negative bacteria are known as narrow spectrum antibiotics.
Example : penicillin.
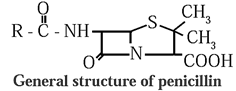
Name and Structure of some antibiotics :
Know This :
|
Traditional knowledge in medicine :
India's ancient medicinal system uses various plants for ailments, leading to a trend in modern chemistry to combine traditional knowledge to isolate active ingredients and develop new drugs.
Example : Turmeric, a native Indian plant contains active ingredient curcumin had been used for centuries for wound healing. Hence, turmeric powder can be used as antiseptic.
Know This : India won the legal battle against US patent and Trademark office (PTO) in 1997 and protected its intellectual property of traditional Indian knowledge about turmeric against patenting.
Active ingredients of some medicinal plants :
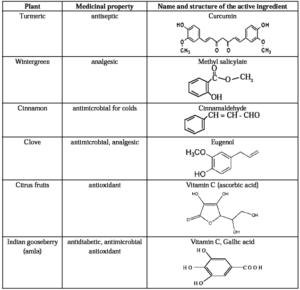
Cleansing Agents :
Cleansing agents are substances which are used to remove stain, dirt or clutter on surfaces. They may be natural or synthetically developed.
Types of cleansing agents :
Commercially cleansing agents are mainly of two types depending upon chemical composition : (i) soaps and (ii) synthetic detergents.
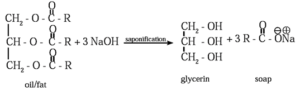
(i) Soaps : Soaps are sodium or potassium salts of long chain fatty acids which contain more than twelve carbon atoms.
Saponification : Soaps are obtained by alkaline hydrolysis of natural oils and fats with NaOH or KOH. This is called saponification reaction.
Chemical Reactions : Soap and glycerol are separated by adding sodium chloride. Soap precipitates out due to common ion effect, and glycerol remains in the solution can be recovered by fractional distillation.
Uses :
- Potassium soaps are softer than sodium soaps. Therefore, toilet soaps are prepared by using better grades of oil and KOH. They are used in shampoo, shaving cream and bathing soaps (liquid or cake).
- Sodium soaps are toilet soaps used for washing purpose.
Remember : We cannot use the same soap for bathing as well as cleaning utensils or washing clothes, because for bathing potassium soaps are used which are soft to skin and soap for cleaning utensils or washing clothes are made using NaOH.
Hard water and soap : Soaps are water soluble. They form scum in hard water and become inactive. This is because hard water contains dissolved salts of calcium
and magnesium, which react with soap, precipitating calcium or magnesium salt of fatty acid (scum) which sticks to fabric.
2R-COONa (aq) + CaCl2(aq) → (R-COO)2Ca(s) + 2NaCl(aq)
Washing soda (Na2CO3) precipitates the dissolved calcium salts as carbonate and helps the soap action by softening of water.
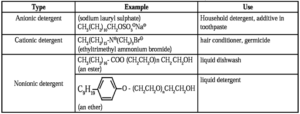
(ii) Synthetic Detergents : Detergents are formulated products containing either sodium salts of alkyl hydrogen sulphates or sodium salts of long chain alkyl benzene sulphonic acids.
There are three types of synthetic detergents, anionic detergents, cationic detergents and nonionic detergents.
(i) Anionic detergents : Anionic detergents are sodium salts of ion chain alkyl sulphonic and they are, prepared from long chain of hydrocarbons or alcohols by treating with conc. H2SO4 and then neutralizing by NaOH.
In anionic detergents, the anionic part of molecule helps in the cleansing action.
Example :
- Sodium dodecyl benzene sulphonate.
- Sodium lauryl sulphate : CH3(CH2)10CH2OSO3—Na+
Use : Anionic detergent is used in tooth paste and in the house hold work.
(ii) Cationic detergents : Cationic detergents are quaternary ammonium salts of long chain alkyl group and a positive charge on nitrogen atom.
They are used as germicides, cetyltrimethyl ammonium chloride is used in hair conditioners.
Examples :
- n—hexadecyl trimethyl ammonium chloride
- CH3(CH2)15 N+ (CH3) 3Br— n-hexadecyl trimethyl ammonium bromide.
(iii) Nonionic detergents : Nonionic detergents are ethers of polyethylene glycol with alkyl phenol or esters of polyethylene glycol with long chain fatty acid.
Examples : CH3(CH2)16-COO (CH2CH2O)n CH2CH2OH
Mechanism of cleansing action :
Dirt is held at the surface by means of oily matter and, therefore, cannot get washed with water. Soaps and detergents effectively clean dirty, greasy surfaces using a mechanism consisting of polar head molecules and a nonpolar tail carbon chain.
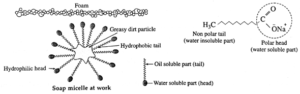
When soap is added to an oily part of cloth or vessel, the nonpolar chain of carbon part of soap dissolves in oil and ionic end of soap dissolves in water. The soap molecules form micelles where one of the molecules is towards the oil droplet while ionic end faces out side. This results in an emulsion in water. The soap micelle assist in dissolving the dirt in water, thus, we can wash our clothes.
Synthetic detergents :
Remember :
- Synthetic detergents are soluble in hard water. They are good cleansing agents can be used in hard water without any wastage. Soaps are insoluble in hard water, so it gets wasted.
- Synthetic detergents can be used in acidic solution while soap precipitates in acidic solution.
- Synthetic detergents are easily soluble in water and produce more lather than soaps.
PDF : Class-11-Chemistry-Chapter-16-Chemistry in Everyday Life-Text Book
PDF : Class-11-Chemistry-Chapter-16-Chemistry in Everyday Life- Notes
PDF : Class-11-Chemistry-Chapter-16-Chemistry in Everyday Life-Solution
All 16 Chapters Notes -11-Chemistry-(16 PDF) Rs.132
All 16 Chapters-Solutions-11-Chemistry- (16 PDF) Rs.128
All 16 Chapters-Notes+Solutions-11-Chemistry- (32 PDF) Rs.228
Main Page : – Maharashtra Board Class 11th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-15 – Hydrocarbons – Online Notes