Respiration and Energy Transfer
Maharashtra Board-Class-11-Science-Biology-Chapter-13
Notes
|
Topics to be Learn :
|
Introduction :
- Maintenance of life requires continuous supply of energy.
- Respiration fulfills the continuous need of energy.
- Nutrients like carbohydrates, fats and proteins are used for energy production
- At cellular level, organisms require energy to carry out different metabolic activities.
- The energy is made available by oxidizing/breaking the food.
- Oxygen is required by aerobic organisms for breaking the food and carbon dioxide is released as a byproduct of oxidation.
Formation of ATP :
ATP :
- ATP (Adenosine triphosphate) is an energy rich organic compound.
- Whenever energy is released, ATP synthesis takes place from ADP and Pi.
- Whenever energy is required for any metabolic reactions, ATP is hydrolysed and energy released as the high energy phosphate bond is broken.
- Therefore, ATP is called energy currency of the cell.
Phosphorylation : Formation of ATP is called as phosphorylation. ATP is formed by addition inorganic phosphate to ADP
ADP + Pi ⇌ ATP
Different ways of Phosphorylation : Phosphorylation occurs in three different ways as - photophosphorylation, Substrate level phosphorylation and oxidative phosphorylation.
Substrate-level phosphorylation : It is a direct phosphorylation of ADP by transfer of a phosphate group from any suitable substrate. It occurs in cytoplasm of the cells and matrix of mitochondria.
Oxidative phosphorylation : In oxidative phosphorylation ATP is synthesize by using the energy released during the oxidation of substrates like NADH + H+ and FADH2. This occurs on the inner mitochondrial membrane only.
Anabolic and catabolic process :
- Anabolic process— Photosynthesis (Biosynthetic process).
- Catabolic process— Respiration (Breakdown process).
Respiration : Respiration is a catabolic process wherein complex organic substrate is oxidized to simple components to generate biological energy. i.e. ATP.
Cellular respiration occurs in two different ways as anaerobic and aerobic respiration.
Anaerobic respiration :
- Anaerobic respiration is the cellular respiration that does not involve the oxygen at all. It is also called as fermentation.
- It involves glycolysis where the product of glycolysis i.e. pyruvate is converted to either lactic acid or ethanol.
Glycolysis :
- Glycolysis is also known as EMP pathway.
- Glycolysis is a process where glucose is broken down into two molecules of pyruvic acid, hence called glycolysis (glucose-breaking).
- It is common to both aerobic and anaerobic respiration.
- It occurs in the cytoplasm of the cell. It involves ten steps.
Fig : Schematic Representation (Glycolysis or EMP Pathway) :

Glycolysis consists of two major phases: (i) Preparatory phase (1-5 steps). (ii) Payoff phase (6-10 steps).
(i) Preparatory phase:
- In this phase, glucose is phosphorylatecl twice by using two ATP molecules and a molecule of fructose 1,6-bisphosphate is formed.
- It is then cleaved into two molecules of glyceraldehyde-3-phosphate and dihydroxy acetone phosphate. These two molecules are 3-carbon carbohydrates (trioses) and are isomers of each other.
- Dihydroxy acetone phosphate is isomerised to second molecule of glyceraldehyde-3-phosphate.
- Therefore, two molecules of glyceraldehyde-3- phosphate are formed.
- Preparatory phase of glycolysis ends.
(ii) Payoff phase:
- In this phase, both molecules of glyceraldehyde-3-phosphate are converted to two molecules of 1,3- bisphoglycerate by oxidation and phosphorylation. Here, the phosphorylation is brought about by inorganic phosphate instead of ATP.
- Both molecules of 1,3-bisphosphoglycerate are converted into two molecules of pyruvic acid through series of reactions accompanied with release of energy. This released energy is used to produce ATP (4 molecules) by substrate-level phosphorylation.
Overall reaction of glycolysis:
Glucose +2ATP + 2iP + 4ADP + 2NAD + → 2Pyruvate + 2ADP + 4ATP + 2NADH + H+ + 2H2O
Reactions of glycolysis :
Irreversible chemical reactions: '
- Some chemical reactions can occur in only one direction i.e. these reactions are irreversible reactions.
- The reactants can change to the products, but the products cannot change back to the reactants.
- Enzymes that catalyse the irreversible reactions : Hexokinase, Phosphofructokinase, Phosphoglycerate kinase and Pyruvate kinase
Reversible chemical reactions:
- Some chemical reactions can occur in both directions i.e. these reactions are reversible reactions. In this case the reactants change to the products and the products can change back to the reactants, at least under specific conditions.
- Out of ten, four are irreversible reactions which involves the enzyme kinase that is required for phosphorylation reactions, these reactions involve large negative energy AG, hence the reactions are irreversible. Other reversible reactions do not involve high negative energy hence are reversible.
Remember :
- Dihydroxy acetone phosphate (DHAP) and. 3-phosphoglyceraldehyde (3-PGAL) are the products of cleavage in glycolysis.
- In glycolysis, dehydration occurs when 2-Phosphoglyceric acid loses a water molecule (dehydration) to form phosphoenol pyruvic acid in presence of the enzyme enolase.
- Glycolysis is only source of energy production in erythrocytes, renal medulla, brain and sperm
- Some plant tissues which are modified to store starch (like potato) mainly depend upon glycolysis for energy production.
Regulation of glycolysis:
- Glycolysis is under tight control. Its rate depends upon the requirement of ATP and many other factors.
- Glycolytic rate control is achieved by complex interplay between ATP consumption, NADH2 regeneration and regulation of various glycolytic enzymes like hexokinase, PFK-1, pyruvate kinase, etc.
- Besides, it is also controlled by hormones like glucagon, epinephrine and insulin.
Role of Mg++, Zn++ in various steps of glycolysis :
- Mg++ and Zn++ are the cofactors that are tightly bound to enzymes and helps the enzymes to perform their functions.
- They regulate the activity of the most important enzymes like Hexokinase, Phosphofmctokinase, Triose phosphate dehydrogenase, Phosphoglycerate kinase, Enolase, Pymvate kinase.
Q. Why is glycolysis considered as biochemical proof of evolution?
- Glycolysis is the first metabolic pathway, an ancient pathway which is common to both aerobic and anaerobic organisms. It does not require oxygen.
- All cells have glycolysis in their metabolic pathway.
- Upto pyruvate the pathway is similar to all aerobic and anaerobic organisms. Later, the fate of pyruvic acid can be either CO2 or ethanol or lactic acid depending upon the type of organism.
Hence it is considered as a biochemical proof of evolution.
Anaerobic respiration in man and yeast :
(i) Anaerobic respiration in man :
In absence of oxygen anaerobic respiration takes place in muscles of man.
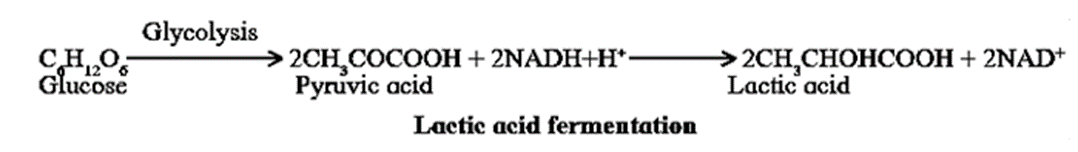
Lactic acid Fermentation :In muscles, the NADH+H+ produced during glycolysis is reoxidized to NAD+ by donating one proton and two electrons to pyruvic acid which yields lactic acid.
- Skeletal muscles usually derive their energy by anaerobic respiration.
- After vigorous exercise lactic acid accumulates, leading to muscle fatigue.
- During rest, however, the lactic acid is reconverted to pyruvic acid and is channeled back into the aerobic respiration pathway.
- White muscle fibres mainly performs anaerobic respiration
Anaerobic respiration in yeast :
- Yeast shows both aerobic and anaerobic respiration depending upon the presence or absence of oxygen.
- In absence of oxygen anaerobic respiration takes place in cytoplasm of the yeast.
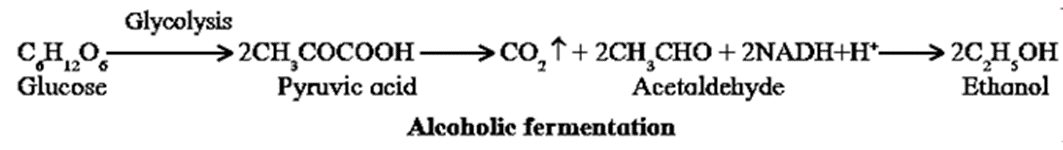
- In absence of oxygen, the pyruvate undergoes anaerobic respiration where it is decarboxylated to acetaldehyde. The acetaldehyde is then reduced by NADH+H+ to ethanol and carbon dioxide. This type of anaerobic respiration is termed alcoholic fermentation.
- Accumulation of ethanol by fermentation in a culture of yeast may stop further multiplication and lead to the death of cells..
- In the presence of oxygen however, it can respire aerobically to produce CO2 and H2O.
Q. Why do athletes like sprinters have higher proportion of white muscle fibers?
- The white muscle fibres produce energy in a very short period of time that is required for fast and severe work. Thus, the energy becomes immediately available to the athletes.
- On the other hand, Red (dark) muscles are richer in myoglobin than the white (pale) muscles. Therefore, red fibers can utilize the oxygen stored in myoglobin to continue energy production over prolonged period by aerobic oxidation of glucose. This enables them to perform sustained work over a long period.
- Hence athletes have higher proportion of white muscle fibres.
Remember :
- In anaerobic respiration, incomplete oxidation takes place which leads to release of less amount of energy.
- Some of the products of anaerobic respiration can be oxidised further to release energy which shows that anaerobic respiration does not liberate the whole energy contained in the respiratory substrate.
- NADH2 does not produce ATP, as electron transport is absent.
- Only 2 ATP molecules are generated from one molecule of glucose during anaerobic respiration.
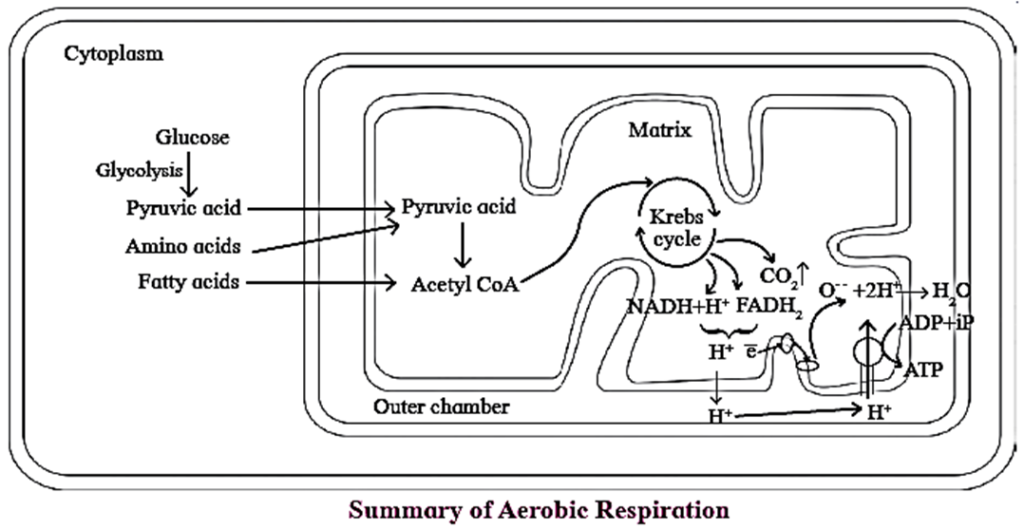
Aerobic Respiration :
- Aerobic respiration involves molecular oxygen during oxidation of glucose, which acts as a final electron acceptor, liberated during oxidation of glucose.
- In this type of respiration, the glucose is completely oxidized to CO2 and H2O with release of large amount of energy.
- It involves glycolysis, acetyl CoA formation (connecting link reaction), Krebs cycle, electron transfer chain reaction and terminal oxidation.
- Aerobic respiration occurs in the mitochorldria in eukaryotes
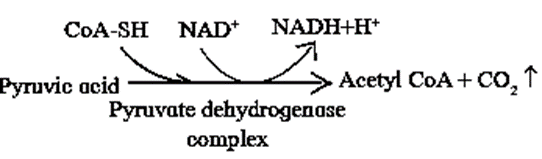
Conversion of pyruvic acid to Acetyl CoA :
- Conversion of pyruvic acid to Acetyl CoA is an oxidative decarboxylation reaction.
- It is catalyzed by multi enzyme complex-pyruvate dehydrogenase complex (PDH). This enzyme is present in mitochondria of eukaryotes and cytosol of prokaryotes.
- This reaction is called as ‘connecting link‘ reaction between glycolysis and Krebs cycle.
Know This :
|
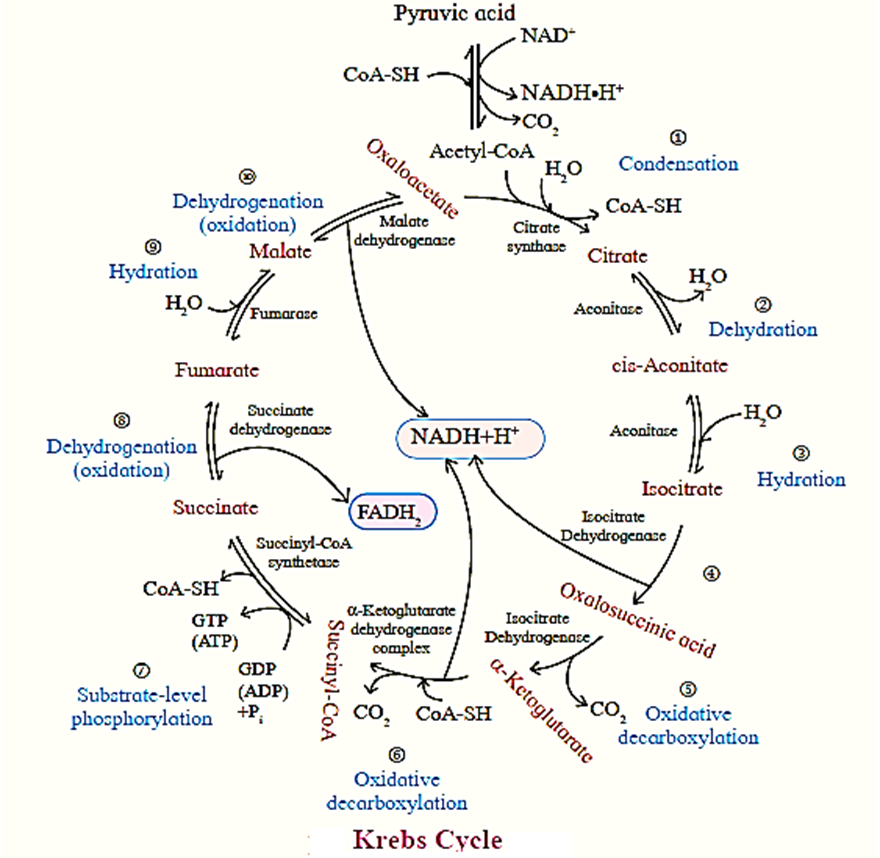
Krebs Cycle ( TCA cycle/ Citric Acid Cycle):
- Krebs cycle or citric acid cycle is the second phase of aerobic respiration which takes place in the matrix of the mitochondria.
- The acetyl CoA formed during the link reaction undergoes aerobic oxidation. .
- This cycle serves a common oxidative pathway for carbohydrates, fats and proteins.
- In mitochondria pyruvic acid is decarboxylated and the remaining 2-carbon fragment is combined with a molecule of coenzyme A to form acetyl-CoA.
- This reaction is an oxidative decarboxylation process and produces H* ions and electrons along with carbon dioxide.
- During the process NAD+ is reduced to NADH+H+.
- B-oxidation of fatty acids also produces acetyl-CoA as the end product.
- Acetyl-CoA from both sources is condensed with oxaloacetic acid to form citric acid. Citric acid is oxidized step-wise by mitochondrial enzymes, evolving carbon dioxide.
- Regeneration of oxaloacetic acid occurs to complete the cycle.
- There are four steps of oxidation in this cycle, catalyzed by dehydrogenases (oxidoreductases) using NAD+ or FAD+ as the coenzyme.
- The coenzymes are consequently reduced to NADH+H+ and FADH2 respectively. These transfer their electrons to the mitochondrial respiratory chain to get reoxidised.
- One molecule of GTP (ATP) is also produced for every molecule of citric acid oxidized.
Significance of Krebs cycle:
- It is a common pathway for breakdown of carbohydrates, proteins and fats, during respiration.
- It forms reduced co-enzymes like NADH2 and FADH2.
- Krebs cycle has the potential to produce 24 ATP from one molecule of glucose.
- The CO2 which is liberated is useful in photosynthesis.
- It forms a number of intermediate products which serve as building blocks for the synthesis of other complex organic compounds
Amphibolic Pathway :
- Respiration is considered as a catabolic process; however, it is not entirely correct in case of Krebs cycle.
- Many reactions of Krebs cycle involve oxidation of acetyl CoA to release energy and CO2.
- However, the breakdown of respiratory substrates provides intermediates like α-ketoglutarate, oxaloacetate are used as precursors for synthesis of fatty acids, glutamic acid and aspartic acid respectively.
- Thus, as the same respiratory process acts as catabolic as well as anabolic pathway for synthesis of various intermediate metabolic products, it is called amphibolic pathway.
Electron Transport chain (Electron transfer system) :
- Wherever the NADH2 (NADH+H+) and FADH2 are produced during glycolysis, connecting link reaction and Krebs cycle, they are oxidised with the help of various electron carriers and enzymes.
- These carriers and enzymes are arranged on inner mitochondrial membrane in the form of various complexes as complex I, II, III, VI and V.
- NADH+H+ is oxidised by NADH dehydrogenase (complex I) and it's electrons are transferred to ubiquinone (coenzyme Q-CoQ) present on inner membrane of mitochondria. Reduced ubiquinone is called as ubiqunol.
- FADH2 is oxidised by complex II (Succinate dehydrogenase) and these electrons are also transferred to CoQ.
- During oxidation of NADH+H+ and FADH2, electrons and protons are released but only electrons are carried forward whereas protons are released into outer chamber of mitochondria (intermembrane space).
- Ubiquinol is oxidised by complex-III (Cytochrome bc 1 complex) and it's electrons are transferred to cytochrome C. Cytochrome C is a small, iron-containing protein, loosely associated with inner membrane. It acts as a mobile electron carrier, transferring the electrons between complex III and IV.

- Cytochrome C is oxidised by complex IV or cytochrome C oxidase consisting of cytochrome a and a3. Electrons are transferred by this complex to the molecular oxygen. This is terminal oxidation.
- Reduced molecular oxygen reacts with protons to form water molecule called as metabolic water.
- Protons necessary for this are channelled from outer chamber of mitochondria into inner chamber by F0 part of oxysome (complex V) present in inner mitochondrial membrane. This proton channelling by F0 is coupled to catalytic site of F1 which catalyses the synthesis of ATP from ADP and inorganic phosphate. This is oxidative phosphorylation. As transfer of protons is accompanied with synthesis of ATP, this process is named as ‘Chemiosmosis' by Peter Mitchell.
Significance of ETS :
- The electron transport system (ETS) or terminal oxidation generates major amount of energy in the form of ATP molecules, 34 ATP molecules out of total 38 ATP molecules are produced through ETS.
- It regenerates oxidized coenzymes such as NAD+ and FAD+ from their reduced forms (NADH+H+ and FADH2) for recycling.
- It also produces water molecules.
- It releases energy in a stepwise manner to prevent damage of cells.
| Know This :
Oxidative phosphorylation :
|
Balance sheet for ATP by aerobic oxidation of 1 glucose molecule.

Glycolysis, TCA cycle and electron transport chain link :
- The coenzymes are initially present in the form of NAD+ and FAD+ which latter get reduced to NADH+H+ and FADH+H+ by accepting the hydrogen from organic substrate during glycolysis, link reaction and Kerbs cycle.
- During glycolysis, glucose is oxidised to two molecules of pyruvic acid with net gain 2 molecules of NADH+H+.
- This pyruvic acid undergoes link reaction to form two molecules of acetyl CoA and two molecules of NADH+H+.
- Acetyl CoA, thus formed enters into the Krebs cycle and it gets completely oxidised to CO2 and H2O with a net gain of 6 NADH+H+ and 2 FADH+H+ are formed.
- During ETS, reduced coenzymes are reoxidized to NAD+ and FAD+ with a net gain of 34 ATPs.
- The ATPs thus formed are used during glycolysis.
- The oxidized NAD+ and FAD+ will again accept the hydrogen from organic substrate. Thus, reduced coenzymes are converted back to their oxidized forms by dehydrogenation to keep the process going.
Respiration Experiment :
Respiration in yeast can be demonstrated with the help of an experiment.
(1) Anaerobic respiration in yeast:
- A pinch of dry baker’s yeast suspended in water containing 10ml of 10% glucose in a test tube (test tube A).
- The surface of the liquid is covered with oil to prevent entry of air and the test tube is closed tightly with rubber stopper to prevent leakage.
- One end of a short-bent glass tube is inserted through it to reach the air inside the tube.
- Other end of the glass tube is connected by a polyethylene or rubber tubing to another bent glass tube fitted into a stopper.
- The open end of the glass tube (delivery tube) is dipped into lime water containing in a test tube (Tube B).
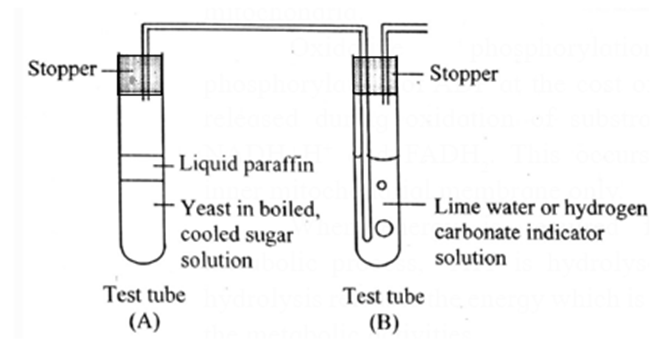
- Stoppers of both the tubes are fitted tightly to prevent leakage of gases. First test tube is placed in warm water (37° C-38° C) in a beaker.
- Lime water gradually turns milky, indicating the evolution of carbon dioxide from the yeast preparation.
- Level of the lime water in the delivery tube does not rise, showing that there is no decline in volume of gas in test tube A and consequently no utilization of oxygen by yeast.
- Preparation is stored for a day or two.
- When we open the stopper of tube A we will notice a smell of alcohol indicating the formation of ethanol.
- From this activity it may be inferred that yeast respires anaerobically to ferment glucose to ethanol and carbon dioxide.
(2) Aerobic respiration in yeast: Experiment explained above can be carried out for demonstrating aerobic respiration in yeast.
- If the level of the lime water in the test tube B rises, indicating intake of oxygen, hence the level of volume of gas rises.
- The preparation tube is stored for a day or two, if no smell of alcohol is noticed it indicates that the yeast respires aerobically.
(3) Respiration in germinating seeds :
- Seed coats of a few germinating seeds (peas, beans or gram) are removed and are then put in a test tube filled with mercury.
- After closing the test tube with the thumb, it is vertically inverted in a trough of mercury and the thumb is carefully removed.
- Being lighter than mercury, the seeds rise to the closed upper end of the test tube.
- No gas is seen at first in the test tube.
- As germination proceeds, a gas begins to collect at the top of the mercury in the test tube.
- On introducing a pellet of potassium hydroxide into the tube, it rises to the top and absorbs the gas. The mercury again fills the tube.
- The potassium hydroxide reacts with carbon dioxide gas to produce potassium carbonate and water.
- The gas therefore disappears. Evidently germinating seeds produce carbon dioxide by anaerobic respiration in the absence of oxygen in the mercury column.
Utility of stepwise oxidation :
Advantage of step wise energy release in respiration :
- In ETS energy is released in step wise manner to prevent damage of cells.
- A stepwise release of the chemical bond energy facilitates the utilization of a relatively higher proportion of that energy m ATP synthesis.
- Activities of enzymes for the different steps may be enhanced or inhibited by specific compounds. This provides a means of controlling the rate of the pathway and the energy output according to need of the cell.
- The same pathway may be utilized for forming intermediates used in the synthesis of other biomolecules like amino acids.
| Remember :
Removal of Hydrogen from respiratory materials is the primary process in respiration :
|
Respiratory substrates : Respiratory substrates are the molecules that are oxidized during respiration to release energy which can be used for ATP synthesis. Carbohydrates, fats and proteins are the common respiratory substrate.
Respiratory Quotient :
Ratio of volume of CO2 released to the volume of O2 consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio. It depends on the type of respiratory substrate.
R.Q. = \(\frac{volume\,of\,CO_2\,released}{volume\,of\,O_2\,consumed}\)
The RQ for different respiratory substrates :
Carbohydrates (R.Q. is 1) :
When carbohydrates are used as substrate, equal volumes of CO2 and O2 are evolved and consumed respectively, thus its R.Q. is 1.
C6H12O6 + 6O2 → 6CO2 + 6H2O
R.Q. = 6CO2/6O2 = 1.0
Fats (R.Q. is less than 1):
Substrates like fats are poorer in oxygen than carbohydrates. Thus, more oxygen is utilized for its complete oxidation.
2(C51H98O6) + 145O2 → 102CO2 + 98H2O + Energy
R.Q. = CO2/O2 = 102/145 = 0.7
Protein respiration (R.Q. is less than 1) :
- When proteins serve as respiratory substrate, they are first degraded to amino acids.
- Then, amino acids are converted into various intermediates of carbohydrates.
- However, amino acids have low proportion of O2 as compared to carbohydrates.
- Thus, they require more O; during their complete oxidation and value of R.Q. becomes less than 1.
- In case of proteins, the R.Q. is approximately 0.9.
Significance of Respiration :
- Respiration provides energy for synthesis of biomolecules.
- It is also a source of energy for cell division, growth, repairs and replacement of worn out parts, movements, locomotion etc.
- Various intermediates of Krebs cycle are used as building blocks for synthesis of other complex compounds.
- Coupled with photosynthesis, it helps to maintain the balance between CO2 and O2 in the atmosphere.
- Anaerobic respiration (fermentation) is used in various industries such as dairies, bakeries, distilleries, leather industries, paper industries etc. It is used in the commercial production of alcohol, organic acids, vitamins, antibiotics etc.
Main Page : – Maharashtra Board Class 11th-Biology – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-12- Photosynthesis : Cockroach – Online Notes
Next Chapter : Chapter-14-Human Nutrition – Online Notes
We reply to valid query.