Carbon and Its Compounds
Class-10-CBSE-NCERT-Science-Chapter-4
Solutions
In−text Solutions (Page 61)
Question 1. What would be the electron dot structure of carbon dioxide which has the formula CO2?
Since, atomic number of C is 6, its electronic configuration would be K-2, L-4.
Similarly, atomic number of oxygen is 8. Hence, its electronic configuration would be K-2, L-6.
To achieve stable electronic configuration of nearest noble gas, carbon needs 4 electrons, while oxygen needs 2 electrons. So, in CO2 molecule, each of oxygen atom share two electrons from carbon. Thus, oxygen and carbon both attain their stable octet. The electron dot structure of CO2 is as follows

Question 2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – The eight atoms of Sulphur are joined together in the form of a ring.)
Since, atomic number of S is 16, its electronic electronic configuration would be K-2, L-8, M-6.
To achieve stable electronic configuration of the nearest noble gas, each sulphur needs 2 electrons. The electron dot structure of S8 is as follows.

In−text Solutions (Page 68)
Question 1. How many structural isomers can you draw for pentane?
Pentane (C5H12) has a skeleton of five carbon atoms which can be arranged in straight chain or in the form of chain containing one or two branches. Molecular formula C5H12 accounts for the following three isomers.
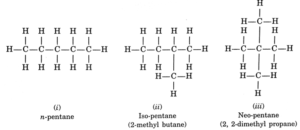
Question 2. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Two main properties which led the carbon to form a huge number of carbon compounds are
- catenation
- tetravalency of carbon
Question 3. What will be the formula and electron dot structure of cyclopentane?
General formula of cycloalkane =CnH2n
In cyclopentane, n = 5, ∴ Formula of cyclopentane, C5H10
Electron dot structure of cyclopentane :
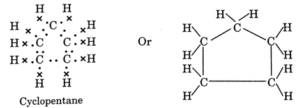
Question 4. Draw the structures for the following compounds.
(i) Ethanoic acid (ii) Bromopentane* (iii) Butanone (iv) Hexanal.
*Are structural isomers possible for bromopentane?

Structural isomers for bromopentane: There are three structural isomers for bromopentane depending on the position of Br at carbon 1, 2, 3. Position 4 and 5 are same as 1, 2.
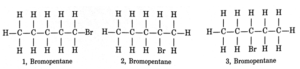
Question 5. How would you name the following compounds?
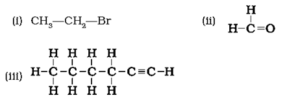
(i) Bromoethane (because of two carbons attach through single bonded, word root is 'eth' and for bromine group, prefix used is 'bromo').
(ii) methanal (because of single carbon, word root is 'meth' and for functional group suffix 'al' is used).
(iii) 1-hexyne (because of 6 carbons, word root is 'hex' and for triple bond, suffix is 'yne'). Also, triple bond is located at first position so position of triple bond is indicated in IUPAC name.
In−text Solutions (Page 71)
Question 1. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Conversion of ethanol to ethanoic acid is oxidation because
- Oxygen is added up to ethanol by oxidising agent like alkaline potassium permanganate or acidified potassium dichromate.
- The product of the reaction is not CO2 and water. Further no heat or light is produced.

Question 2. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Ethyne with oxygen gives enough heat that can be used for welding whereas if it is burnt in air which contains nitrogen and other inactive gaseous contents, sufficient oxygen is not available for burning ethyne to give the required heat.
2CH≡CH + 5O2 -> 4CO2 + 2H2O + Heat + Light
In−text Solutions (Page 74)
Question 1. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Alcohol and carboxylic acid can be distinguished experimentally by two reactions:
(i) Reaction with base Alcohols do not react with base like NaOH, KOH while carboxylic acids react with base to form salt and water.
e.g. CH3COOH + NaOH → CH3COONa + H2O
CH3CH2OH + NaOH → No reaction
(ii) Reaction with sodium bicarbonate Carboxylic acids react with sodium bicarbonate rapidly to give brisk effervescence of CO2 gas while alcohols do not react.
e.g. CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 ↑
CH3CH2OH + NaHCO3 → No reaction
Question 2. What are oxidising agents?
Oxidising agents are substances which give oxygen to another substance.
Example : Alkaline potassium permanganate or acidified potassium dichromate can give oxygen to alcohols to convert them into carboxylic acids.
In−text Solutions (Page 76)
Question 1. Would you be able to check if water is hard by using a detergent?
No, because detergent does not form scum with hard water.
Question 2. People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine.
Why is agitation necessary to get clean clothes?
- When soap solution is made and dirty clothes are soaked in it, then micelles formation occurs. This results in the formation of an emulsion (a stable suspension of small droplets of one liquid in another with which is immiscible).
- To wash away the loosen dirt particles in the form of micelles from the surface of the cloth, it is either scrubbed mechanically or beaten or agitated in washing machine.
Exercise Solutions
Question 1. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
(b) 7 covalent bonds.
Question 2. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
(c) ketone.
Question 3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
(b) the fuel is not burning completely.
If the bottom of the vessel is getting blackened, it means that the air holes are blocked and fuel is getting wasted as it is not burning completely.
Question 4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Covalent bond/s is formed by sharing of electrons so that the combining atom/s complete their outermost shell. In CH3Cl, this happens as follows:
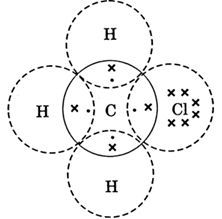
- Three hydrogen atoms complete (K = 1 + 1 = 2) their shells by sharing three electrons (one electron each) of carbon atom.
- Chlorine completes its outer shell (L = 7 + 1 = 8) by sharing its one out of seven electrons with one electron of carbon atom.
- Thus carbon atom shares in all its four electrons with three of three hydrogen atoms and one of chlorine atom and completes its outer shell (L = 4+3+1 = 8).
Question 5. Draw the electron dot structures for
(a) ethanoic acid. (b) H2S. (c) propanone. (d) F2.

Question 6. What is an homologous series? Explain with an example.
A series of similarly constituted compounds in which the members present have the same functional group, similar chemical properties and any two successive members in a particular series differ in their molecular formula by (-CH2) unit, is called a homologous series.
e.g. Alkane series CnH2n+2
CH4 - Methane
C2H6 - Ethane
C3H8 - Propane
C4H10 - Butane
C5H12 – Pentane
Question 7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
(i) Distinctions based on physical properties are as
(a) Smell Ethanoic acid has a pungent smell while ethanol has a pleasant smell.
(b) Melting point Ethanol has lower melting point (156 K) than ethanoic acid (290 K).
(c) Physical state Ethanoic acid is solid (glacial acetic acid) in winters but ethanol is always a liquid.
(ii) Distinctions based on chemical properties are as :
(a) Reaction with sodium hydrogen carbonate On adding a small amount of sodium hydrogen carbonate to ethanoic acid, carbon dioxide gas is evolved with brisk effervescence. However, no such reaction takes place in case of ethanol.
CH3COOH + NaHCO3 → CH3COONa + CO2 ↑ + H2O
C2H5OH+ NaHCO3 → No reaction
(b) Reaction with base Ethanoic acid reacts with both sodium hydroxide (NaOH) and potassium hydroxide (KOH) to form corresponding salt and water. Ethanol fails to react with either of these.
CH3COOH + NaOH → CH3COONa + H2O
CH3COOH + KOH → CH3COOK + H2O
Question 8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
- When soap is at the water's surface, the hydrophobic 'tail' is insoluble in water, and the soap aligns along the surface, with the ionic end in water and the hydrocarbon 'tail' extending out of water.
- Inside water, these molecules have a distinct orientation that keeps the hydrocarbon part out. This is accomplished by arranging molecules in clusters with hydrophobic tails in the inside and ionic ends on the exterior. This structure is known as a micelle.
- Micelle does not develop in all types of solvent. It will form in solvents in which soap is soluble.
- g. Soap is insoluble in ethanol, hence, no micelle formation takes place.
Question 9. Why are carbon and its compounds used as fuels for most applications?
- Carbon burns in oxygen (air) to form carbon dioxide and water.
- During this reaction, a large amount of heat and light is released.
- Further once ignited, carbon and its compounds keep on burning without the requirement of additional energy. Hence, they are used as fuels.
C + O2 → CO2 +Heat +Light
Question 10. Explain the formation of scum when hard water is treated with soap.
When soap is added to a sample of hard water, calcium and/or magnesium ions present in hard water react with soap forming insoluble calcium/magnesium soap which is a sticky and greasy mass (called scum) and thus no lather is formed.

Question 11. What change will you observe if you test soap with litmus paper (red and blue)?
Soap is alkaline in nature. Hence, it turns red litmus paper blue and have no effect on the blue litmus paper.
Question 12. What is hydrogenation? What is its industrial application?
Hydrogenation : The addition of hydrogen to unsaturated hydrocarbons in the presence of catalyst is known as hydrogenation.
Industrial application : When hydrogen gas is made to pass through vegetable oil in the presence of nickel catalyst, it changes to solid fat (ghee).
Vegetable oils + H2 \(\frac{Nickel}{Heat}→\) Fat (ghee)
Question 13. Which of the following hydrocarbons undergo addition reactions:
C2H6, C3H8, C3H6, C2H2 and CH4.
Unsaturated hydrocarbons like alkenes and alkynes (containing double and triple bond respectively) undergo addition reactions and their general formula are CnH2n and CnH2n-2 respectively.
Thus, C3H6, and C2H2 will undergo addition reactions.
Question 14. Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
- Butter contains saturated compounds while cooking oil contains unsaturated compounds.
- Since, unsaturated compounds are oxidised by alk. KMnO4 with disappearance of its pink colour.
- Therefore, when cooking oil is treated with a few drops of alk. KMnO4, pink colour of KMnO4 With butter, however, the pink colour of KMnO4 does not disappear.
Question 15. Explain the mechanism of the cleaning action of soaps.
- The cleansing action of soaps and detergents is due to their capacity to reduce the surface tension of water and retain its suspension in water. When soap is dissolved in water is hydrophobic ends attach themselves to dirt and remove it from the cloth.
- Soap solution appear cloudy because the ion aggregate to form spherical clusters which forms micelles that are large enough to scatter light.
Click on below links to get PDF from store
PDF : Class 10th-Science-Chapter-4-Carbon and Its Compounds-Notes
PDF : Class 10th-Science-Chapter-4-Carbon and Its Compounds-Solution
Main Page : NCERT-Class-10-Science – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-3- Metals and Non-Metals – Online Solutions
Next Chapter : Chapter-5- Life Processes – Online Solutions
We reply to valid query.