Metals and Non-Metals
Class-10-CBSE-NCERT-Science-Chapter-3
Notes Part-2
Topics to be learn : Part-2
|
Ionic compounds :
The compounds that can conduct electricity in water or in molten state are ionic compounds. These are crystalline solids having high melting points.
Properties of ionic compounds :
The important characteristic properties of Electrovalent compounds are :
- Ionic compounds consist of ions: All ionic compounds consist of positively and negatively charged ions and not molecules. In the crystal of sodium chloride every sodium ion is surrounded by six evenly spaced chloride ions and vice-versa in a regular fashion. Similarly, in potassium nitrate, the units of the crystal are the potassium and the nitrate ions.
- Ionic compounds are solids: Because of strong electrostatic attractions between ions, ionic compounds are solids and relatively hard. But these compounds are generally brittle and break into pieces when pressure is applied.
- Ionic compounds have high m. pts and b. pts: Due to the powerful electrostatic force between the ions in a crystal of an ionic/electrovalent compound, considerable energy is needed to overcome these forces and breakdown the crystalline lattice. Hence these compounds possess high melting pts. and boiling pts.
- Ionic compounds are generally soluble in water: Polar solvents like water weaken the interionic attractions and break the lattice. Therefore, ions are dispersed and dissolve in water and are insoluble in non-polar solvents like kerosene, acetone, petrol etc.
- Ionic compounds conduct electricity in molten state or when dissolved in water: When an electrovalent compound is melted or dissolved in water, the binding forces in the crystal disappear and the component ions become mobile and they conduct electricity, whereas in solid form, ions are fixed in a lattice and thus the salt does not conduct electricity in solid form.
Formation of MgCl2 by transfer of electrons :
In the formation of MgCl2 two electrons from magnesium are transferred to two chlorine atoms. Magnesium atom becomes Mg2+ and chlorine atom becomes Cl−. Then one Mg2+ and two Cl− ions are held together with strong attractive forces.

Q. How many aluminium atoms would combine with oxygen atoms to form aluminium oxide? Explain.
- An aluminium atom (2, 8, 3) loses 3 electrons to achieve noble gas configuration of neon gas and forms an aluminium cation (2, 8) having three positive charges.
- An oxygen atom (2, 6) attains noble gas configuration of neon gas after accepting two electrons and forms an oxide anion with two negative charges.
- As the final compound is electrically neutral, the positive charges in aluminium oxide compound must be balanced by equal number of negative charges. This is possible when 2 aluminium cations (6+ charge) combine with 3 oxide anions (6− charge).
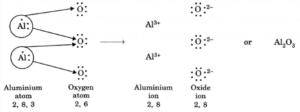
- Thus, the ratio between aluminium cations and oxygen anions to form aluminium oxide is 2:3. This is indicated by the chemical formula 2Al3+·3O2− or Al2O3.
Occurrence of Metals :
Mineral: A mineral is a naturally occurring inorganic solid that has a definite composition and a definite structure. Talc and granite are minerals.
Ore: An ore is defined as a mineral from which a metal can be extracted economically.
- A rock or a natural product serving as a source of non-metallic elements like sulphur and fluorine is also called an ore.
Gangue: Ores are associated with earthy and rocky materials as impurities. These impurities are called gangue or matrix.
Occurrence of metals :
Free state: A few metals occur native or free in the earth.
- Gold, platinum and silver are some of the metals occurring in the free state.
- They have low chemical reactivity and are not affected by the action of the chemicals or atmospheric conditions.
- These are the metals at the bottom of activity series.
Combined state: The metals having appreciable reactivity are found in the combined state.
- They occur as oxides, halides, sulphides, phosphates, silicates etc.
- The highly electropositive elements (Na, K, Mg, etc.), are found as compounds of highly electronegative elements (chlorine, oxygen etc.).
- Weakly electropositive (Fe, Cu) metals are found in combination with weakly electronegative elements (sulphur).
- Many metal ores are found as oxides. This is because oxygen is very reactive and very abundant on earth.
Metallurgy : The extraction of a metal from a suitable ore and its refining for use is called metallurgy or metallurgical operations.
Types of ores :
Ores are classified into the following types:
- Native ores: Silver, gold, platinum etc., are found in native state in the earth’s crust.
- Oxide ores: An ore in which a metal occurs as an oxide is called an oxide ore. For example, bauxite (Al2O3.2H2O), haematite (Fe2O3), pyrolusite (MnO2).
- Sulphide ores: An ore in which a metal occurs as sulphide is called a sulphide ore. For example, Iron pyrites (FeS), Galena (PbS), Copper pyrites (CuFeS2), Cinnabar (HgS).
- Carbonate ores: An ore in which a metal occurs as carbonate or basic carbonate is called a carbonate ore. For example, limestone (CaCO3), dolomite (CaCO3, MgCO3).
- Halide ores: Aluminium occurs as a fluoride, e.g., cryolite (Na3AlF6). Sodium and potassium occur as chloride, e.g., carnallite (KCl, MgCl2.6H2O), rocks salt (NaCl).
- Sulphate ores: Barium and lead occur as barytes (BaSO4) and anglesite (PbSO4) respectively.
Form of pure non-metals exist in nature :
- Because of the tendency of the atoms of non-metals to achieve noble gas configuration by covalent bonding, similar atoms tend to combine and thus pure non-metals exist in a variety of molecular stages of aggregation.
- For example; chlorine atoms combine to form a chlorine molecule which is a gas; two bromine atoms combine to form a bromine molecule which being heavier exists as liquid; eight sulphur atoms combine to form S which exists as a solid.
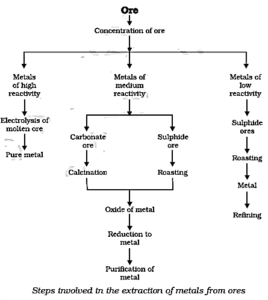
Metallurgical operations :
Metallurgical operations mainly involve four steps:
(1) Enrichment of ores: Ores are found mixed with many impurities like earthy, materials, rocky material, sand, limestone, mica etc. These impurities are known as gangue.
- Removal of gangue is based on the differences between physical or chemical properties of ore and the gangue.
- The separation of gangue from the ore of a metal is called concentration of ore. Following methods may be used for this purpose. However, all these processes are not used for all metals.
- Depending on the chemical composition of the ore and impurities associated with it, suitable methods may be chosen:
(i) Hydraulic washing: In hydraulic washing, the advantage of differences in density is taken into account. The lighter gangue particles are washed away in a stream of water while the heavier minerals stay behind. Ores of tin and lead are very heavy so that they are concentrated by this method.
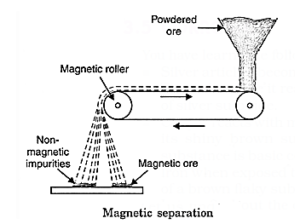
(ii) Magnetic separation: Some ores are attracted by a magnet whereas gangue is not attracted. The magnetic ores like iron pyrites, FeS and magnetite, Fe3O4 are concentrated by this method as shown below in Fig.The crushed ore is allowed to pass through electromagnetic belts. The mineral particles are retained and gangue particles are thrown away.
(iii) Froth flotation process: This method is employed for the concentration of sulphide ores. The finely powdered ore is added to water in a tank containing pine or eucalyptes oil. The mixture is agitated by blowing a blast of air. The ore particles preferentially wetted by oil come to the surface with froth while impurities which are wetted with water become heavier and settle at the bottom (see Fig.).
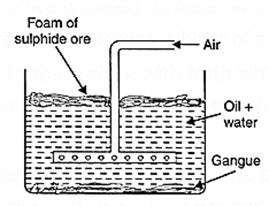
The oil froth containing mineral particles which floats on the surface of the water can be skimmed off easily. Thus, the concentration of copper pyrite and galena etc., are done by this method. .
(iv) Chemical separation: In this method, use is made of differences between chemical properties of minerals and gangue. For example, ore of aluminium (bauxite) can be separated from its gangue by this method. Bauxite (Al2O3) is treated with hot sodium hydroxide solution. Sodium aluminate formed is soluble in water and can be easily separated from gangue by simple filtration. Aluminium hydroxide is then precipitated on acidifying the filtrate. Al2O3 is obtained from aluminium hydroxide on heating.
(2) Conversion of concentrated ore into oxide: It is easier to obtain metals from their oxides than from sulphides or carbonates. Therefore, sulphide and carbonate ores are converted into oxides as follows:
(i) Calcination: It is the process of heating the concentrated ore in the absence of air. By this process, volatile impurities are removed.
Carbonate and hydrated oxide ores are calcined as shown.
Al2O3.2H2O \(\underrightarrow{\text{Heat}}\) Al2O3 + 2H2O
Bauxite
CaCO3 \(\underrightarrow{\text{Heat}}\) CaO + CO2 ↑
Limestone
(ii) Roasting: It is the process of heating the ore in the presence of excess of air. This method is used in case of sulphide ores.
The sulphides are converted into oxides by roasting and SO2 is evolved. For example,
2ZnS + 3O2 \(\underrightarrow{\text{Heat}}\) 2ZnO + 2SO2 ↑
Zinc Blende
2PbS + 3O2 \(\underrightarrow{\text{Heat}}\) 2PbO + 2SO2 ↑
(Galena)
(3) Reduction of metal oxide to metal: There are three ways of reducing metal compounds mainly metal oxides to metals.
(i) Reduction on heating : Metals low in the activity series can be reduced by heating their compounds. For example, mercury can be obtained by heating HgS (cinnabar) in air. This process is also called roasting of ore. However, metals higher up in the activity series give oxides on roasting.
HgS + O2(g) → Hg + SO2(g)
2Cu2S + 3O2(g) → 2Cu2O(s) + 2SO2(g)
(ii) Reduction by metals: Metals in the middle of activity series, e.g., iron, zinc, nickel, tin etc., can be reduced by heating with carbon. The process of reducing the oxide with coke (carbon) is called smelting. For example, iron oxide is reduced to metal by heating with carbon.
ZnO + C → Zn + CO
Fe2O3 + 3C → 2Fe + 3CO
Sometimes displacement reactions are used. The highly reactive metals such as sodium, potassium, calcium, aluminium etc., can be used as reducing agents because these can displace metals of lower reactivity from their compounds. Cr and Mn are obtained by the reduction of their oxides by aluminium powder.
For example, manganese oxide is reduced as follows to give manganese metal.
3MnO2 + 4Al → 3Mn + 2Al2O3
These reactions are highly exothermic. The heat evolved is enough to give the reduced metal in the molten form. This process is known as thermite reaction.
(iii) Electrolytic reduction: The oxides of more reactive metals like sodium, potassium and aluminium are very stable and cannot be reduced by chemical reduction. Such metals are extracted by the electrolysis of their oxides, hydroxides or chlorides in the molten state.
For example, aluminium metal is extracted by the electrolysis of molten aluminium oxide and sodium by electrolysis of fused sodium chloride.
(4) Refining or purification of the impure metal: The metals produced by various reduction processes are not very pure and contain a lot of impurities. The most widely used method for refining impure metals is electrolytic refining.
Electrolytic refining: Many metals like copper, nickel, silver etc., are refined by this method. The impure copper is refined in the following way.
The impure copper metal is made the anode of an electrolytic cell (Fig.). The cathode is made of a thin plate of pure copper.The electrolyte is a solution of copper salt in water, e.g., copper sulphate.
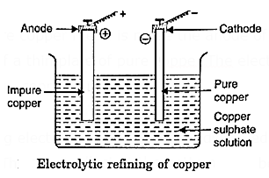
On passing electric current, copper is dissolved from the anode and deposited on the cathode. The impurities collect at the bottom below the anode which are known as anode mud.
Steps involved in the extraction of metals from their ores :
Corrosion :
- When the surface of a metal is attacked by air, water and some other substance it is said to corrode. The phenomenon is known as corrosion.
- When iron is exposed to moist air for a long time, its surface acquires a coating of a brown, flaky substance. The brown surface easily peels off the iron surface, which if exposed further to moist air again acquires more of that brown layer. This is due to corrosion of iron in moist-air.
- The flaky substance formed is called rust. Rust is mainly hydrated ferric oxide-
- Fe2O3.xH2O.
- Another example is copper metal. The surface of copper in moist air acquires a green coating of basic copper carbonate, Cu(OH)2.CuCO3.
- Metals which corrode easily are: iron, copper
- Metals which do not corrode are: gold, platinum.
- Air and water both are necessary for corrosion of iron.
Effect on some metals if exposed to air :
| Metal | Compound formed on exposure to air | Colour change |
| Silver | Silver sulphide | Black |
| Copper | Basic copper carbonate CuCO3.Cu(OH)2 | Green |
| Iron | Ferric oxide, iron hydroxide Fe2O3.xH2O, Fe(OH)3 | Reddish Brown |
Q. Aluminium corrodes in moist air but it is widely used for making cooking vessel and other cutlery. Explain.
Aluminium does corrode with the formation of thin layer of aluminium oxide sticking to the surface. This layer of aluminium oxide is non-reactive and thus protects the metal underneath from further damage.
Prevention of Corrosion :
Corrosion of metals can be prevented by the following methods:
- Barrier protection: In this method, the surface is coated with some impermeable layer such as paint or oil greasing to prevent the access of damp air.
- Electroplating: The surface is coated electrolytically with a metal which will not be oxidised easily. e.g., electroplating of nickel, chromium, tin etc., on articles to be protected.
- Sacrificial protection: In this case coated metal gets corroded in preference to the one which is protected. For example, iron sheets are coated with zinc (Galvanisation). Here even if the coating gets punctured, zinc will be preferentially oxidised.
- Cathodic protection: This is the most elegant method. The metal to be protected (say an iron tank) is made the cathode either by connecting to external power source or to a more active metal like magnesium.
Galvanisation : It is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc. The galvanised article is protected against rusting even if the zinc coating is broken, because even if the coating gets punctured, zinc will be preferentially oxidised.
Method of improving the properties of a metal :
Alloying : The process of alloying is a very effective method for improving a metal's properties. By using this technique, we can obtain the desired properties.
An alloy is a homogeneous mixture of two or more metals, or a metal and a nonmetal.
- Properties of any metal can be changed if it is mixed with some other substance.
- The substance added may be a metal or a non-metal.
- It is prepared by first melting the primary metal, and then, dissolving the other elements in it in definite proportions. It is then cooled to room temperature.
Examples :
Iron : Iron is the most widely used metal. But it is never used in its pure state. This is because pure iron is very soft and stretches easily when hot.
- If iron is mixed with a small amount of carbon (about 0.05 %), it becomes hard and strong.
- When iron is mixed with nickel and chromium, we get stainless steel, which is hard and does not rust.
- Thus, if iron is mixed with some other substance, its properties change.
Gold : Pure gold, known as 24 carat gold, is very soft. It is, therefore, not suitable for making jewellery. It is alloyed with either silver or copper to make it hard.
In India, 22 carat gold is used for making ornaments. It means that 22 parts of pure gold is alloyed with 2 parts of either copper or silver.
| Know This : |
In alloy, if one of the metals is mercury, then the alloy is known as an amalgam. The electrical conductivity and melting point of an alloy is less than that of pure metals.
- Brass, an alloy of copper and zinc (Cu and Zn), and bronze, an alloy of copper and tin (Cu and Sn), are not good conductors of electricity whereas copper is used for making electrical circuits.
- Solder, an alloy of lead and tin (Pb and Sn), has a low melting point and is used for welding electrical wires together.
Click on below links to get PDF from store
PDF : Class 10th-Science-Chapter-3-Metals and Non-Metals-Text Book
PDF : Class 10th-Science-Chapter-3-Metals and Non-Metals-Notes
PDF : Class 10th-Science-Chapter-3-Metals and Non-Metals-Solution
Main Page : NCERT-Class-10-Science – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-2- Acids, Bases and Salts – Online Notes
Next Chapter : Chapter-4- Carbon and its Compounds – Online Notes
We reply to valid query.