Carbon and Its Compounds
Class-10-CBSE-NCERT-Science-Chapter-4
Notes
|
Topics to be learn :
|
Introduction :
Carbon : A Versatile Element :
- The versatile element carbon is the basis of many items we use in daily life.
- Carbon serves as the basis for all living things.
- Carbon is the basic ingredient of our body. Millions of molecules ranging from the small and simple methane molecule to the extremely big D.N.A. molecule are made from carbon.
- The crust of the earth and the atmosphere both contain very little carbon. Only 0.02% of carbon is present in the earth's crust in the form of minerals, while 0.03% of carbon dioxide is present in the atmosphere.
- Despite the fact that there is just a small quantity of carbon in nature, it seems to have a huge impact.
BONDING IN CARBON – THE COVALENT BOND :
Type of carbon atom bond :
The electronic configuration of carbon is K(2), L(4). Thus it has 4 electrons in its outermost shell. In order to react or to achieve noble gas configuration, it should either gain or lose 4 electrons.
- It cannot gain 4 electrons and become C4− anion because its nucleus with 6 protons cannot hold on 10 electrons.
- Also it cannot lose 4 electrons and become C4+ because a very large amount of energy would be required to remove four electrons from a small atom like carbon having 3 very close nucleus with 6 protons.
Therefore Carbon does not form ionic compounds. Carbon overcomes above problem and forms a large number of compounds by sharing its valence electrons with other atoms of carbon or with atoms of other elements called covalent bond, and thereby satisfying requirement to attain noble configuration of its own and of all the combining atoms.
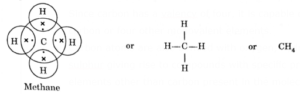
Example : carbon forms methane as shown below: (Dots and crosses depict valence electrons of carbon and hydrogen respectively.)

In methane, carbon shares its four valence electrons with four valence electrons, one each from four hydrogen atoms.
Thus carbon attains the configuration (2, 8) as that of nearest noble gas, neon. And all four hydrogen atoms individually attain the configuration of helium gas (K = 2). Bond so formed due to sharing of electrons is called covalent bond.
Covalent bond : The covalent bond is said to be formed when two atoms achieve stability by sharing of an electron pair, each contributing one electron to the electron-pair. In this way the atoms can be regarded as having acquired a noble gas configuration.
Examples :
(i) Formation of chlorine molecule: Two chlorine atoms share one electron of each and acquire stable electronic configuration as shown:
Chlorine forms a diatomic molecule, Cl2

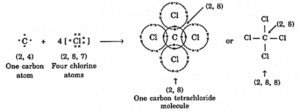
(ii) Formation of carbon tetrachloride:
- The outermost shell of a carbon atom contains 4 electrons.
- Carbon tetrachloride is formed by sharing 4 electrons with 1 electron from each of 4 chlorine atoms.
- Four chlorine atoms then share 1 electron with each carbon atom.
- Thus, 1 carbon atom and 4 chlorine atoms complete their octet by electron sharing, and carbon tetrachloride with 4 covalent bonds is created as shown:
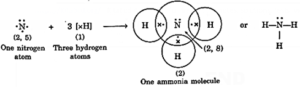
(iii) Formation of ammonia molecule:
- Nitrogen (N) atom has 5 electrons (2, 5) in the outermost shell.
- It shares one electron each of three hydrogen atoms to form ammonia molecule as shown.
- One unshared pair of electrons in ammonia molecule is not involved in bond formation and is called a lone pair of electrons.
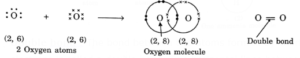
Double bond : The bond between two atoms formed by sharing of two pairs of electrons is called a double bond. Two horizontal lines between two atoms denote a double bond, e.g. O=O (O2).
Examples :
(i) Formation of oxygen molecule, O2: Oxygen atom has six electrons in its outermost shell, two short of forming a complete octet. Thus each oxygen atom in O2 shares 2 electrons with the other oxygen atom and attains stable configuration as shown:
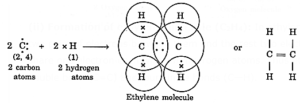
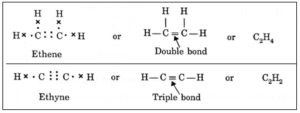
(ii) Formation of ethylene molecule (C2H4): In ethylene two carbon atoms share two pairs of electrons between them and the rest two electrons on each carbon atom share one electron each with two hydrogen atoms. Thus ethylene molecule has one double bond (C=C) and four single bonds (C—H).
Triple bond or triple covalent bond :
- The bond formed between two atoms through the sharing of three electron pairs is called a triple bond.
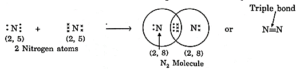
- Three horizontal lines between two atoms denote a triple bond. e.g., nitrogen molecule is given as N≡N.
Examples :
(i) Formation of N2 molecule: Nitrogen atoms have five electrons in their outermost shells. Therefore, in N2 molecules, two nitrogen atoms share 3 electrons with each other, thus acquiring the stable structure and forming a N2 molecule with triple bonds.
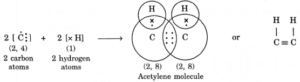
(ii) Formation of acetylene molecule (C2H2): In acetylene molecule, each carbon atom satisfies its valency by sharing one electron w1th a hydrogen atom and sharing the other three electrons with the other carbon atom as shown:
Physical properties of Covalent compounds (carbon compounds) :
Carbon forms covalent compounds which exhibit the following properties:
- Covalent compounds have strong bonds within the molecule but intermolecular forces are very weak and so have low melting pts. and boiling pts.
- Since the electrons are shared between atoms and no charged particles are formed, such covalent compounds are generally poor conductors of electricity. They do not conduct electricity either in the solid state or in molten state or in the solution form. They are non-electrolytes.
- These are readily soluble in non-polar solvents like benzene, carbon tetrachloride etc., but do not dissolve in polar solvents like water.
- The covalent bond is directional in nature.
Allotropy : When an element possesses two or more different crystalline forms in the same state, they are called allotropes and the phenomenon is known as allotropy.
Allotropes of carbon which occur in nature are as given below :
- Diamond and graphite are the two allotropes of carbon. They are chemically identical.
- When equal quantities of diamond and graphite are burnt, both of these produce the same amount of carbon dioxide.
- However, as they exist in different forms in the solid state, they have entirely different physical properties.
Physical properties of diamond and graphite :
| Property | Diamond | Graphite |
| Appearance | Transparent | Shining black |
| Hardness | Very hard | Soft, slippery to touch |
| Electrical conductivity | Poor conductor | Very good conductor |
| Thermal conductivity | Very poor | Moderate |
| Density, kg/m3 | 3,510 | 2,250 |
VERSATILE NATURE OF CARBON :
Carbon : A Versatile Element : Carbon has two unique features:
- It has the property of catenation.
- It is tetravalent.
The above two features enables carbon to form a large number of compounds with varying size, shape.
- Carbon has the unique property to form bonds with other atoms of carbon, i.e., carbon atoms have long chains or even are arranged in branches or rings.
- Carbon atoms in its compounds are linked by single, double or triple bonds.
- Since carbon has a valency of four, it is capable of bonding with four atoms of carbon or four other monovalent elements.
- Carbon atoms are also bonded with oxygen, hydrogen, chlorine, nitrogen and sulphur giving rise to compounds with specific properties typical of the elements other than carbon present in the molecule.
Carbon atom exhibit property of catenation :
- Because of the small size of the carbon atom, its nucleus can strongly hold the shared pair of electrons.
- Because of this, carbon forms strong bonds with the majority of other elements, and the compounds formed are extremely stable when compared to covalent bonds formed by other elements.
Saturated hydrocarbons:
A hydrocarbon in which each carbon atom is attached to four other atoms, is known as saturated hydrocarbon. The bonds so formed are single covalent bonds. These hydrocarbons are also called alkanes.
Example : Methane
Unsaturated hydrocarbons:
Hydrocarbons containing either a carbon—carbon double bond (C=C) or a carbon—carbon triple bond in their molecules are called unsaturated hydrocarbons.
Examples: Ethene, Ethyne
Classification of hydrocarbons :
Hydrocarbons can be classified either on the basis of nature of bonds or nature of chains between carbon atoms.
(a) On the basis of bonds : Hydrocarbons are classified as
- Saturated hydrocarbons, e.g., CH4 (methane).
- Unsaturated hydrocarbons, e.g., C2H4 (ethene).
(b) On the basis of chains: Hydrocarbons are classified as
- Aliphatic hydrocarbons: They have straight or branched chains of carbon atoms, e.g., butane, isobutane.
- Cyclic hydrocarbons: These contain rings of carbon atoms, e.g., cyclohexane, benzene.
Know This : Other than carbon, silicon forms compounds which have chains like carbon, exhibits the property of catenation upto 7 or 8 atoms. Silicon compounds are few and are comparatively less stable.
Name and structure of saturated hydrocarbons having 3, 4, 5 and 6 carbon atoms joined in a straight chain :
Propane, butane, pentane and hexane for saturated hydrocarbons containing 3, 4, 5 and 6 carbon atoms respectively.

Alkyl radical : The group formed by removal of the hydrogen atom from an alkane molecule is called an alkyl radical or alkyl group.
For example, if hydrogen is removed from methane, it is —CH3 (methyl) group.
Functional groups :.A group which determines the chemical nature of an organic compound is called a functional group.
- For example, Cl in CH3Cl, OH in CH3OH are functional groups.
- Functional groups are the groups, other than hydrocarbon part, present in carbon compounds
- Compounds having similar functional groups show similar chemical behaviour.
- Their physical properties may differ.
.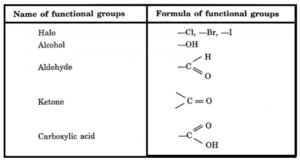
Homologous series : A homologous series is a group or family of compounds which contains the same functional group but have different chain lengths. These chains can be branched also. Thus, these have the same chemical properties but different physical properties that vary in a regular manner.
Characteristics of a homologous series are:
- It has a general formula in terms of number of carbon atoms.
- It has the same functional group, if any.
- The members of a homologous series, i.e., homologous, have similar chemical properties.
- Various homologous can be prepared by the general method of preparation for the series.
- Two successive (adjacent) homologous differ by 1 carbon atom and 2 hydrogen atoms in their molecular formulae.
- The members of a homologous series show a gradual change in their physical properties with increase in molecular mass.
- Melting point and boiling point of hydrocarbons increase with increase in molecular mass.
Examples:
(i) The homologous series of alkanes : Single bond Carbon compound, General Formula CnH2n+2 Examples :CH4, C2H6, C3H8, C4H10
(ii) The homologous series of alkenes : The unsaturated hydrocarbons containing a carbon-carbon double bond are called alkenes. e.g. Ethene (CH2 = CH2), General Formula CnH2n Examples : C3H6, C4H8, C5H10
- M.Pts and B.Pts of C5H10 is higher than C4H8 because molecular weight of C5H10 is more than that of C4H8 in the same homologous series.
- Increasing B.Pts : C3H6 < C4H8 < C5H10
(ii) The homologous series of alkynes : The unsaturated hydrocarbons containing a carbon-carbon triple bond are called alkynes. e.g. Ethyne (CH ≡ CH), General Formula CnH2n-2 Examples : C3H4, C4H6, C5H8
Two consecutive aldehydes: Acetaldehyde(CH3CHO) and Propaldehyde(CH3CH2CHO)
- Physical properties: Hydrocarbon part, i.e., CH3— and CH3CH2— determine their physical properties.
- Chemical properties: Aldehyde part, i.e., —CHO determines their chemical properties. This is the same in both acetaldehyde and propaldahyde and so these compound have similar chemical properties.
Homologous series of alcohols : CH3OH, C2H5OH, C3H7OH, C4H9OH etc..
Next homologue of CH3OH : CH3CH2OH
Next homologue of HCOOH : CH3COOH
Next homologue of CH3CH2OH is propanol (C3H7OH).
Next homologue of HCOOH is acetic acid (CH3COOH)
Isomers : The compounds that contain the same molecular formula but different structures are called structural isomers. The isomers of a compound have different physical properties.
For example, butane with the molecular formula, C4H10 may exist in the following two isomeric forms:

Isomerism : The phenomenon in which two or more compounds having same molecular formula may exist with different structural formulae is called isomerism. For example : n-butane and isobutane have same molecular formula, C4H10 but exhibit different structural formula.
Remember : It will be seen that no matter how the atoms in methane, ethane and propane are arranged, structural formulae of these always remain the same. Therefore it is not possible to have isomers of methane, ethane and propane.

Cyclic hydrocarbons : Cyclic hydrocarbons are saturated or unsaturated organic compounds that contain rings of carbon atoms e.g., cyclohexane C6H12 (saturated), benzene C6H6 (unsaturated) etc.
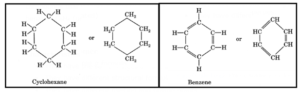
Characteristics of isomers:
- They have the same molecular formula.
- They have different structural formulae.
- They have different melting and boiling points
- They have different chemical properties.
Nomenclature of hydrocarbons :
(1) The number of carbon atoms in a hydrocarbon (or any other organic compounds) is indicated by using the following stems:
- One carbon atom is indicated by writing ‘Meth’
- Two carbon atoms is indicated by writing ‘Eth’
- Three carbon atoms is indicated by writing ‘Prop’
- Four carbon atoms is indicated by writing ‘But’
- Five carbon atoms is indicated by writing ‘Pent’
(2) Straight chain hydrocarbons :
- A straight chain saturated hydrocarbon containing single bonds is indicated by writing the word ‘ane’ after the stem. For example, a four carbon atom saturated hydrocarbon (C4H10) is called butane and a 5-C atom straight saturated hydrocarbon is called pentane (C5H12).
- An unsaturated hydrocarbon containing a double bond is indicated by writing the word ‘ene’ after the stem. The structure of C3H6 is as follows;

It contains 3 carbon atoms and a double bond. So its IUPAC name is ‘Propene’
- (iii) An unsaturated hydrocarbon containing a triple bond is indicated by the word ‘yne’ after the stem. The structure of C3H4 is
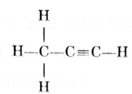
It contains 3 carbon atoms and a triple bond. So its IUPAC name is ‘Propyne'.
(3) Branched chain saturated hydrocarbons: The following rules should be followed for naming branched chain saturated hydrocarbons:
- First find out the longest chain of carbon atoms in the structure of the compound.
- The compound is then named on the basis of number of carbon atoms present in the longest chain. (This is called parent hydrocarbon).
- The methyl groups present as side chains are considered substituents and named separately, e.g., methyl (CH3—) or ethyl (C2H5—) groups.
- The carbon atoms of the parent hydrocarbon are numbered in such a way that the alkyl groups (substituents) get the lowest possible number. That is, the terminal carbon atom which is nearest to the carbon atom containing an alkyl group (or a substituent) is given number 1.
- The position of the alkyl group is indicated by writing the number of carbon atom to which it is attached.
- The IUPAC name of the compound is obtained by writing the ‘position and name of alkyl group’ just before the name of the parent hydrocarbon.
Example: One of the branched chain structures of C5H12 hydrocarbon is given below:
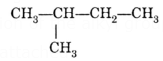
Because the longest carbon chain has four carbon atoms, this is a butane derivative. The long chain carbon atoms are now numbered beginning with the terminal carbon atom closest to the carbon atom connected to a methyl group. As a result, C-atoms are numbered from left to right in this situation. Its IUPAC name is 2-methyl butane

(4) Hydrocarbons containing functional groups: In case a functional group is present, it is indicated in the name of the compound with a prefix (for halides) and a suffix for other functional groups. If the name is to be given by a prefix, then halo (chloro, bronio, iodo) is written before the name of the corresponding hydrocarbon, e.g., chloropropane is CH3CH2CH2Cl. If the name of the functional group is to be given by a suffix, the name of the carbon chain is modified by deleting the final ‘e’ and substituting the appropriate suffix.
For example, a two carbon chain with an aldehyde group would be named as:
(i) Ethane - e = ethan + ‘al’ = ethanal
(ii) A three carbon chain with a ketone group
Propane — e = propane + ‘one’ = Propanone
(iii) A three carbon chain with an alcoholic group
Propane — e = propan + ‘ol’ = Propanol
(iv) If the carbon chain is unsaturated then final ‘ane’ in the name of carbon is substituted by ‘ene’ or ‘yne’; a three carbon chain with a double or triple bond.
Propane — ane = prop + ‘ene’ = propene (double bond)
Propane — ane = prop + ‘yne’ = propyne (triple bond)
Examples of IUPAC names :
(i) IUPAC name of straight chain hydrocarbon C5H12: C5H12 is saturated straight chain hydrocarbon (CnH2n+2). It contains 5 carbon atoms. So its IUPAC name is pentane.
CH3-CH2—CH2—CH2—CH3
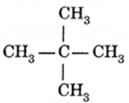
(ii) IUPAC name of branched chain saturated hydrocarbon (C5H12). The parent hydrocarbon i.e., the longest chain contains 3 carbon atoms. So it is named as derivative of propane. It has 2 side chains or methyl groups. When carbon atoms are numbered from left or right side both the methyl groups appear at carbon atom numbered 2. So its IUPAC name is 2, 2-dimethyl propane
(iii) IUPAC name of C4H8: C4H8 is an unsaturated hydrocarbon and is alkene. It has one double bond and contains four carbon atoms. So its IUPAC name is butene.
CH3-CH2—CH=CH2
(iv) IUPAC name of C4H6: C4H6 is an unsaturated hydrocarbon. It contains four carbon atoms and a triple bond. So its IUPAC name is butyne.
CH3-CH2—CH≡CH2
Concept of functional group: Functional groups are important in organic chemistry for the following reasons:
- Functional groups are used to name organic compounds.
- Organic compounds are classified based on the functional groups they contain.
- Chemical reactions take place at functional groups.
- Compounds with similar functional groups exhibit similar chemical behaviour.
Nomenclature of alkyl halides :
- The general formula of alkyl halides is CnH2n+1.X, where X is the halogen atom (F, Cl, Br, I).
- Longest carbon chain is chosen and the alkyl halide is named by adding the prefix: Fluoro (F), Chloro (Cl), Bromo (Br) and Iodo (I) before the alkane of the main chain.
- The position of halogen atom is indicated by a number assigned to the carbon atom to which it is attached.
- The prefixes di- or tri- are used if 2 or 3 halogen atoms of the same element are attached to the main carbon chain.
Example : CH3CHBr2, BrCH2CH2Br
(i) The functional group in CH3CHBr2 is bromine.
(ii) Since it has two C-atoms and 2-Br atoms, it should be named as dibromo ethane.
(iii) Both the bromine atoms are attached to the same C-atom and so the carbon chain should be numbered from the C-atom attaching bromine atoms. The structure of CH3CHBr2 is
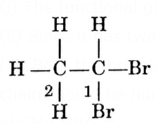
Common name: Ethylidene dibromide.
IUPAC name: 1, 1 dibromo ethane.
IUPAC name of BrCH2CH2Br: 1, 2 dibromo ethane
Alcohols : The hydroxyl group (—OH) when present in aliphatic organic compounds is known as an alcoholic group. The corresponding compound is an alcohol. An alcohol is named in IUPAC system by replacing —e of the corresponding alkane by the suffix —ol.
Examples:
| Hydrocarbon | IUPAC name | Common name | Formula |
| Methane | Methanol | Methyl alcohol | CH3OH |
| Ethane | Ethanol | Ethyl alcohol | C2H5OH |
| Propane | Propanol | Propyl alcohol | C3H7OH |
| Butane | Butanol | Butyl alcohol | C4H9OH |
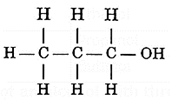
Structure of an alcohol with three carbon atoms :
Name : Propyl alcohol : C3H7OH
Structural formula :
Aldehydes :
An organic compound containing or —CHO as functional group is called an aldehyde.
An aldehyde is named by replacing -e of the parent alkane by the suifix -al. Note that the carbon atom of the aldehyde group is also to be counted for finding the longest carbon chain. The carbon atom of aldehyde group is numbered 1.
For example,
(1) HCHO is named as methanal.
METHAN [E] + AL → METHANAL
(2) CH3CHO
- The functional group in CH3CHO is the aldehyde group (—CHO)
- CH3CHO contains 2 atoms and is, therefore, named as derivative of ethane.
- Since CH3CHO is an aldehyde, the suffix e of ethane is replaced by the suffix -al
- The IUPAC name of CH3CHO is Ethanal.
- The common name of CH3CHO is Acetaldehyde.
Ketone :
The group ![]() is called a ketonic group. The carbon atom of a ketonic group is bonded to an oxygen atom by a double bond and to two carbon atoms of two alkyl groups by single covalent bonds. The general formula of a ketone is
is called a ketonic group. The carbon atom of a ketonic group is bonded to an oxygen atom by a double bond and to two carbon atoms of two alkyl groups by single covalent bonds. The general formula of a ketone is
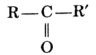
where R and R’ are any alkyl groups
Important : An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. With molecular formula C3H6O, two compounds are
(i) CH3CH2CHO : Propaldehyde (Aldehyde) and
(ii) Acetone (Ketone) :
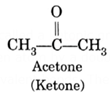
- Relations : These are isomeric to each other
Simplest ketone : The simplest ketone is dimethyl ketone or acetone. The structural formula of acetone is
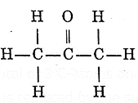
It contains total of 3 C-atoms and it is the derivative of propane. Since it is a ketone, the suffix -e is replaced by the suffix -one. Therefore, IUPAC name is propanone.
C2H5COCH3 :
- The functional group in C2H5COCH3 is carbonyl group or
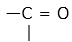 ketonic group
ketonic group - C2H5COCH3 contains a total of 4 carbon atoms and is therefore to be named as derivative of butane, C4H8.
- Since the compound is a ketone, the suffix -e of butane is replaced by suffix -one. Therefore, the IUPAC name of C2H5COCH3 is Butanone.
Carboxylic acids :
General formula of carboxylic acid is R—COOH. They are named by replacing suffix ‘e’ of corresponding alkane as given from the total number of carbon atoms by ‘oic’ and then adding acid at the end.
Examples:
| Formula | Common name | Name (IUPAC) |
| HCOOH | Formic acid | Methanoic acid |
| CH3COOH | Acetic acid | Ethanoic acid |
| C2H5COOH | Propionic acid | Propanoic acid |
Molecular, electronic, structural formula of acetic acid :
- IUPAC name : Ethanoic acid
- Functional group : —COOH
- Molecular formula of acetic acid : CH3COOH (C2H4O2)
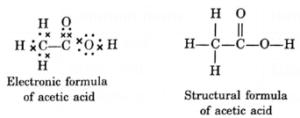
CHEMICAL PROPERTIES OF CARBON COMPOUNDS
Combustion : Combustion is the burning of compounds in air to give CO2 and water with the liberation of heat and light.
For example,
(i) Carbon compounds on combustion (complete oxidation) give CO2 and H2O.
CH4(g) + 2O2 → CO2(g) + 2H2O(g)
Combustion reactions of carbon and its compounds :
Carbon, its allotropic forms and compounds burn in sufficient oxygen to give carbon dioxide and water, with the liberation of large amount of heat and light.
C + O2 → CO2 + heat + light
CH4 + 2O2 → CO2 + 2H2O + heat + light
CH3CH2OH + 3O2 → 2CO2 + 3H2O + heat + light
If carbon or its compound are burnt in an insufficient supply of air, then incomplete combustion of carbon or its compound takes place and carbon monoxide and water vapour and some heat are produced.
2C4H10 + 9O2 \(\frac{incomplete}{combustion}→\) 8CO(g) + 10H2O(g) + heat
Combustion reactions of saturated and unsaturated hydro carbons :
Saturated hydrocarbons will generally give a clean flame while unsaturated carbon compounds will give a yellow flame with lots of black smoke. This results in a sooty deposit on the metal plate
- Saturated hydrocarbons burn with a blue flame because these have high percentage of hydrogen
- Unsaturated hydrocarbons on burning leave excess carbon deposits and burn with sooty flame.
Q. Kerosene burns in a cooking stove to give a blue flame while it gives a yellow flame when burnt in a lantern. Give reasons for this difference.
Ans. In a kerosene lamp or lantern, there is no adequate supply of oxygen and carbon particles remain unburnt due to incomplete combustion and they glow in the flame and flame appears yellow.
In a wick stove a perforated metal sheet is used around the Wicks. The perforations allow hot air to enter near the wick and complete combustion of kerosene vapour takes place, and no unburnt particle remains, so the colour of flame is blue.
Oxidation : Oxidation is the reaction in which carbon compounds take up oxygen in the presence of oxidising agents to give another carbon compound. Thus all combustion reactions are also oxidation reactions but oxidation reactions are not combustion reactions. In fact combustion means complete oxidation.
Examples :
(i) Alcohols are oxidised to acids in the presence of alkaline potassium permanganate or acidified potassium dichromate. Ethanol oxidized to Ethanoic acid
CH3CH2OH \(\underrightarrow{O}\) CH3COOH
(ii) With cold dilute alkaline potassium permanganate, ethene (ethylene) is oxidised to ethylene glycol.
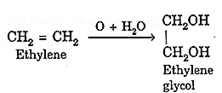
Chemical properties of hydrocarbons:
(i) Combustion in air:
Hydrocarbons burn in air and react with oxygen gas to give carbon dioxide and heat.
These reactions are highly exothermic but unsaturated hydrocarbons and aromatic hydrocarbons give sooty flame.
CH4 + 2O2 → CO2 + 2H2O + heat + light
CH3CH2OH + 3O2 → 2CO2 + 3H2O + heat + light
(ii). Reactions with halogens:
(a) Saturated hydrocarbons are inert, they do not react with bromine. However, they do react with chlorine in the presence of light or at high temperature. These are called substitution reactions because the hydrogen atoms are substituted by chlorine atoms.
CH4 + Cl2 → CH3Cl(Methyl Chloride) + HCl
Further chlorination gives CH2Cl2 (methylene dichloride), CHCl3 (chloroform) and CCl
(carbon tetrachloride) respectively.
(b) The unsaturated hydrocarbons undergo additional reactions. They are more reactive because of the tendency of the doubly bonded carbon atoms to become saturated. Bromine adds to the unsaturated hydrocarbons. For example,
CH2 = CH2 + 2Br2 → CH2Br—CH2Br
Similarly, bromine reacts with acetylene.
HC≡CH + 2Br2 → CHBr2—CHBr2
- When few drops of bromine (which is brown in colour) are added to ethylene, bromine is used up by the double bond of ethylene and brown colour of bromine disappears and colourless ethylene dibromide is formed.
Any compound which docolo11risos bromine is an unsaturated compound. However, saturated hydrocarbons, e.g., ethane do not add up bromine and so colour of bromine is not decolourised when ethane and bromine are mixed.
(iii) Reaction with hydrogen:
(a) Saturated hydrocarbons do not react at all with hydrogen.
(b) Unsaturated hydrocarbons react with hydrogen by adding in the presence of metal catalyst like finely divided platinum, palladium or nickel. The reaction is called catalytic hydrogenation or addition reaction.
CH2=CH2 + H2 \(\frac{catalyst}{Ni}→\) CH3—CH3 Or C2H6
CH≡CH + 2H2 \(\frac{catalyst}{Ni}→\) CH3—CH3
Addition Reaction : The process of adding hydrogen to unsaturated hydrocarbons is called addition reaction.
- Unsaturated hydrocarbons add hydrogen in the presence of catalysts such as palladium or nickel to give saturated hydrocarbons.
- In addition reaction, an atom or group of atoms are added up to an unsaturated carbon compound.
Examples :
(a) Ethylene adds up chlorine to form ethylene dichloride.
CH2 = CH2 + Cl2 → CH2Cl—CH2Cl
(b) Ethylene adds up hydrogen to form ethane in the presence of palladium or nickel catalyst.
CH2 = CH2 + H2 \(\underrightarrow{Ni}\) CH3—CH3
Use :
- Vegetable oils can be converted into vegetable ghee by addition of hydrogen in the presence of catalyst. The purpose of catalyst is to enhance the reaction.
Oils + H2 \(\underrightarrow{Ni}\) Ghee
Know This : Animal fats generally contain saturated fatty acids which are said to be harmful for health. Vegetable oils contain less fat and so considered as healthy.
Substitution Reaction
Saturated hydrocarbons are fairly unreactive and are inert in the presence of most reagents. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen atoms one by one. It is called a substitution reaction because one type of atom or a group of atoms takes the place of another. A number of products are usually formed with the higher homologues of alkanes
CH4 + Cl2 → CH3Cl + HCl (in the presence of sunlight)
SOME IMPORTANT CARBON COMPOUNDS – ETHANOL AND ETHANOIC ACID
Ethanol : Ethanol is a liquid at room temperature (156 K and 351 K are melting and boiling points of ethanol). Ethanol is commonly called alcohol and is the active ingredient of all alcoholic drinks.
Physical properties of alcohols :
- State: Most common alcohols are liquids.
- Electrical conductivity: Like hydrocarbons they do not conduct electricity.
- Solubility: Lower members of the series, e.g., methanol, ethanol, propanol are quite soluble in water. As the number of carbon increases, the solubility readily decreases.
- Action on sodium: They react with sodium to produce hydrogen.
- Action of heat: They readily burn like hydrocarbons.
- Boiling point: Boiling point of alcohols are higher than the hydrocarbons of the same molecular weight.
Production of Ethanol/ethyl alcohol :
- Ethyl alcohol on a large scale is obtained by the fermentation of molasses (obtained from sugarcane juice) with enzymes (invertase and zymase) present in the yeast.
- Fermentation is carried out at 20—30°C in a vessel which allows the escape of carbon dioxide formed during the process but prevents air from entering into it.
- During fermentation, sugar and starch molecules are broken down into smaller molecules containing ethanol with evolution of carbon dioxide gas.
- Ethyl alcohol is separated by fractional distillation.
C12H22O11 + H2O \(\underrightarrow{invartase}\) C6H12O6 + C6H12O6
Cane sugar → Glucose + Fructose
C6H12O6 → 2C2H5OH + 2CO2
Glucose/fructose → Ethyl alcohol + Carbon dioxide
The liquid froths during fermentation because of formation of carbon dioxide.
Important uses of ethyl alcohol :
- It is a vital solvent for many organic compounds.
- It is used to sterilise wounds and syringes.
- It is used as a solvent in laquers, varnishes, perfumes, and medicines, among other things.
- It is used in the production of paints, dyes, medicines, and a wide range of organic compounds.
- It is used in the production of antifreeze mixtures that are used in the radiators of motor vehicles in cold countries because water mixed with ethyl alcohol has a much lower freezing point than water alone.
- It is used for alcoholic beverages such as whisky, wine, beer, and other liquors.
- It can also be used as a fuel.
Fermentation : It is a slow process of breaking down of organic compounds by microorganisms to simpler molecules. It is an exothermic process and is carried out at 20—30°C.
Examples:
(i) Production of ethyl alcohol from molasses (cane sugar)- Yeast
C12H22O11 + H2O \(\underrightarrow{Yeast}\) 4C2H5OH + 4CO2.
(ii) Production of acetic acid from fermented liquors (15% ethyl alcohol)
CH3CH2OH \(\frac{Bacterium}{Acetic}→\) CH3COOH
Ethyl alcohol → Acetic acid
Remember : Ethyl alcohol contains hydroxyl group (—OH) which gets bonded to water molecules readily through hydrogen bonding. So it is miscible with water in all proportions.
Harmful effects of alcohol on human beings:
- It is an intoxicant. Under its influence, a person loses mental balance to distinguish between right and wrong.
- Excess of alcoholism affects lungs of a person and may lead to his death.
- It makes a person addicted to its use.
- Its consumption causes continuous economic loss to the family.
- If a person takes alcohol adulterated with methanol or pyridine, he is liable to lose his eye-sight.
Denatured alcohol : Ethyl alcohol is a part of drinking beverages. It is also used widely as a solvent in industries. To make it unsuitable for drinking, certain poisonous substances like pyridine, methyl alcohol and copper sulphate (dye) are added to it. This is called denatured alcohol.
Methylated spirit is ethyl alcohol to which some methyl alcohol has been added to make it undrinkable.
Reactions of Ethanol
Reaction with sodium : When a piece of sodium metal is poured over ethanol, vigorous reaction occurs and hydrogen gas is bubbled out.
2C2H5OH + 2Na → 2C2H5ONa + H2 ↑
Alcohols react with sodium leading to the evolution of hydrogen. With ethanol, the other product is sodium ethoxide. The gas evolved, i.e., H2 burns with a pop second when exposed to burning matchstick which extinguishes.
Reaction with acetic acid (ethanoic acid) :
Ethyl alcohol (C2H6O) reacts with acetic acid (ethanoic acid) in the presence of a few drops of conc. sulphuric acid to form a sweet smelling substance called ester (ethyl acetate/Ethyl ethanoate). Sulphuric acid acts as catalyst. Such a reaction is called esterification.
C2H5OH + CH3COOH \(\underrightarrow{Conc.H_2SO_4}\) CH3COOC2H5 + H2O
Saponification : The reaction of an ester to react with an acid or base to give back the alcohol and carboxylic acid is called saponification. This is so-called because this reaction is used in the preparation of soap.
Uses of esters:
- Esters are used in perfumes.
- Esters are used for preparation of soaps and detergents
Reaction to give unsaturated hydrocarbon: Heating ethanol at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene
CH3CH2-OH \(\underrightarrow{Hot\,Conc.H_2SO_4}\) CH2=CH2 + H2O
The concentrated sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
Oxidation of ethyl alcohol (Ethanol) :
(i) Oxidation of ethyl alcohol with cupric oxide: Copper oxide acts as a mild oxidising agent. Ethyl alcohol vapour when passed through a tube packed with CuO at 200-300°C gets oxidised into acetaldehyde.
CH3CH2OH + CuO \(\underrightarrow{Heat}\) CH3CHO + Cu + H2O
(ii) Oxidation of ethyl alcohol with acidified potassium dichromate or potassium permanganate: When ethyl alcohol is heated with a mixture of potassium dichromate or potassium permanganate and dilute sulphuric acid, its complete oxidation takes place and acetic acid is formed,
CH3CH2OH + 2[O] \(\underrightarrow{H_2SO_4+K_2Cr_2O_7}\) CH3COOH + H2O
Q. Why is ethyl alcohol used as a fuel in spirit lamp?
Ans. Ethyl alcohol burns in air with a non-luminous flame to form carbon dioxide, steam and a large amount of heat is given out. Therefore, it is used as a fuel in spirit lamp.
CH3CH2OH + 3O2 → 2CO2 + 3H2O + Heat
Q. Why is a mixture of water and alcohol used instead of water in radiatorsof vehicles in cold countries?
Ans : Water freezes at 0°C. Thus in cold countries where the temperatures falls below 0°C, water cannot be used in radiators. Therefore, a mixture of water and alcohol is used because (i) Water and alcohol are miscible in all proportions and (ii) a mixture of water and alcohol has much lower freezing point than that of water.
Distinguish between ethanol and ethanoic acid.
- Ethanol shows no change/reaction with either litmus paper or sodium hydrogen carbonate.
- Ethanoic acid solution in water turns blue litmus red. It reacts with –sodium hydrogen carbonate with effervescence and gives out carbon dioxide gas which turns lime water milky.
Uses of acetic acid or ethanoic acid:
- It is used as vinegar for preparing pickles.
- It is used for the preparation of aspirin used for relieving headache.
- It is used for preparing cellulose acetate which is an important artificial fibre.
- It is used to coagulate rubber from latex.
Know This : 5-8% solution of acetic acid is called vinegar. Glacial acetic acid is pure acetic acid. Its m.pts. is 290 K and often it freezes during winter, hence ethanoic acid got its names as glacial acetic acid.
SOAPS AND DETERGENTS
Soap : Soap is the sodium (Or potassium) salt of a higher fatty acid like palmitic, oleic or stearic acid.
Examples :
- Sodium palmitate(C15H31COONa)
- Sodium oleate (C16H33COONa)
- Sodium stereate (C17H35COONa)
Soaps are molecules in which two ends have different properties,
- One end is hydrophilic that is it interacts with water,
- Other end is hydropholic, i.e. it interacts with hydrocarbons only.
Soap with hard water :
- When soap is added to a sample of hard water, calcium and/or magnesium ions present in hard water react with soap forming insoluble calcium/magnesium soap which is a sticky and greasy mass (called scum) and thus no lather is formed.
Ca2+ + 2C17H35COONa → (C17H35COO)2Ca + 2Na+
- When soap is used with hard water for cleaning clothes, a part of it is used in removing hardness as an insoluble calcium and magnesium soap. After the removal of all calcium and magnesium ions, the soap then forms lather which cleans the clothes. Thus, lot of soap is wasted in removing hardness, when hard water is used for cleaning clothes.
Preparation of soap in the laboratory: Boil about 10 ml of castor oil or soyabean oil (or any other plant oil or animal fat) with 10 ml of 40% sodium hydroxide in a beaker till a thick paste is obtained.
Then add a small amount of water glass or sodium carbonate to harden the paste. Soap is finally precipitated out from the aqueous solution by the addition of sodium salt. The soap, thus separated is then mixed with a desired colouring agent and perfume and cast into various shapes for use.
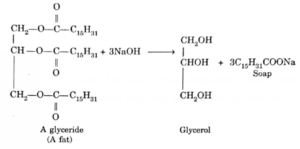
(Fat gets decomposed into glycerol and soap. The process of splitting the fat is called saponification)
Cleansing action of soaps :
Dirt is generally absorbed in the clothes as an oily material. It cannot be removed with water because it does not mix well with water. But when a cloth with dirt is soaked in soap solution, the dirt and grease attach themselves to the hydrocarbon component (e.g., C15H31—) of the soap molecule. The —COONa part of the soap which is attached to thewater molecules pulls the hydrocarbon part along with dirt away from the surface of the cloth, thus washing it clean.
Micelles :
Soaps are molecules in which the two ends have differing properties, one is hydrophilic, that is, it interacts with water, while the other end is hydrophobic, that is, it interacts with hydrocarbons. When soap is at the surface of water, the hydrophobic ‘tail’ of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ protruding out of water. Inside water, these molecules have a unique orientation that keeps the hydrocarbon portion out of the water. Thus, clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle.
Soap in the form of a micelle is able to clean, since the oily dirt will be collected in the centre of the micelle. The micelles stay in solution as a colloid and will not come together to precipitate because of ion-ion repulsion. Thus, the dirt suspended in the micelles is also easily rinsed away.
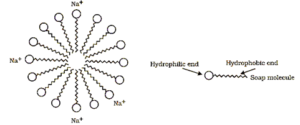
The soap micelles are large enough to scatter light. Hence a soap solution appears cloudy.
Know This :
- Soaps help to make the grease or other oily matter soluble with the formation of water soluble micelles. This property of soaps called Emulsifying action.
- Sodium sulphate or sodium silicate. are added to make the soap dry
- Ordinary soaps hydrolyse in water and give NaOH which causes irritation to skin.
- Soaps cannot be used in acidic water because these get precipitated as insoluble free acids and adhere to fabrics, thereby even preventing their drying.
- Since soap is alkaline, its solution will turn red litmus blue.
Synthetic Detergents :
Detergents are the sodium salts of a long chain benzene Sulphonic acid or a long chain alkyl hydrogen sulphate. Most of the detergents have C8 to C12 hydro carbon chains (a non-polar group) and a polar group SO3−Na+.
For example, Sodium n-dodecyl benzene sulphonate and sodium n-dodecyl sulphate (or sodium lauryl sulphate)
(i) Sodium lauryl sulphate :
CH3—(CH2)10—CH2SO4
(ii) Sodium n-dodecyl benzene sulphonate.
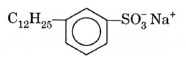
- Synthetic detergents made with linear hydrocarbons are more easily degraded by microorganisms present in Water bodies like lakes and rivers. Hence, these do not cause much water pollution and are more acceptable.
Ordinary washing soap and detergents : Ordinary washing soap (natural detergent) does not form lather in hard water or water containing Ca++ or Mg++ ions and washing of clothes becomes difficult. Synthetic detergents form lather even with hard water and wash clothes well
Explanation :
- The structure of synthetic detergents is similar to that of soaps. They have a long hydrocarbon chain which is water repelling, and a short ionic part which is water attracting.
- The water attracting groups in a synthetic detergent are usually a sodium sulphonate group (SO3−Na+.) or sodium sulphate group (SO4−Na+.).
- The cleansing action of both soaps and detergents are similar. Thus, chemically these are not different. Difference lies only in the hydrocarbon part.
- Synthetic detergents are made from long chain hydrocarbons obtained from petroleum whereas hydrocarbon part of soaps are of vegetable oils.
- In soaps, water attracting part is —COONa whereas in detergents it is —SO3Na.
- Detergents are preferred to soaps because these form lather even with hard water and have better cleansing action. These do not form scums with Ca++ or Mg++ ions.
Advantages of synthetic detergents:
- Use of vegetable oils (which can be used as food articles) is minimised
- They can be used even with hard water.
- They have better cleansing properties.
Disadvantages of synthetic detergents:
- Use of synthetic detergent creates water pollution because they are not easily bio-degradable, i.e., decomposed by microorganisms.
- Use of detergents involves diminishing petroleum products.
Difference between soap and detergent :
| soap | detergent |
| When used with hard Water a lot of it is wasted in removing Ca2+/Mg2+ salts as curdy precipitate. | It forms good lather with hard water and thus can be safely used even with hard water. |
| It is completely oxidised to CO2 by bacteria present in sewage and so it does not create any pollution problem in rivers. | Its excess creates pollution problem in rivers since it is not fully bio-degradable. |
| It is obtained easily from edible oil. | It is obtained from costly petroleum products. |
Know This : Temporary hardness can be removed if hard water is heated with sodium salt or boiled.
Click on below links to get PDF from store
PDF : Class 10th-Science-Chapter-4-Carbon and Its Compounds-Notes
PDF : Class 10th-Science-Chapter-4-Carbon and Its Compounds-Solution
Main Page : NCERT-Class-10-Science – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-3- Metals and Non-Metals – Online Notes
Next Chapter : Chapter-5- Life Processes – Online Notes
We reply to valid query.