Respiration and Energy Transfer
MHTCET Exam-Biology-Chapter-2
Notes
Introduction :
- Maintenance of life requires continuous supply of energy.
- Respiration fulfills the continuous need of energy.
- Nutrients like carbohydrates, fats and proteins are used for energy production
- At cellular level, organisms require energy to carry out different metabolic activities.
- Oxygen is required by aerobic organisms for breaking the food and carbon dioxide is released as a byproduct of oxidation.
Photosynthesis converts light energy into chemical energy, stored in chemical bonds between complex organic substances like carbohydrates, proteins, fats, and organic acids.
Oxidation breaks down these energy-rich substances, releasing energy for various activities. This essential catabolic process, known as respiration, occurs in all living organisms' cells. The term 'respiration' was coined by Dutrochet.
Respiration comprise of two phases. First phase is gaseous exchange between environment and organism through stomata and lenticels in plants or through special respiratory organs in higher animals and humans and the second phase is cellular respiration.
Cellular Respiration :
It is an intracellular catabolic process of oxidation-reduction reaction, in which the complex organic food materials are broken down to form simpler end products with the stepwise release of energy, i.e. ATP and carbon dioxide.
Respiratory substrates are organic molecules oxidized during respiration to release energy, with carbohydrates being the main source, followed by fat, the proteins used in special circumstances only.
Efficiency of cellular respiration is 45%. 114.5 kcal energy is obtained by oxidation of each oxygen molecule.
Formation of ATP
ATP (Adenosine triphosphate) is an energy rich organic compound. Whenever energy is released, ATP synthesis takes place from ADP and Pi.
ATP acts as the energy currency in the cell. This energy is utilised in various energy-requiring processes of the organisms such as division, growth, movements, metabolism, reproduction, etc. and the carbon skeleton produced during respiration is used as precursors for biosynthesis of other molecules in the cell.
The formation of ATP is called as phosphorylation. In nature it occurs via three ways as
- Photophosphorylation,
- Substrate level phosphorylation
- Oxidative phosphorylation.
Out of these three, photophosphorylation occurs during photosynthesis and rest occurs during respiration.
- Substrate level phosphorylation is a direct phosphorylation of ADP by transfer of a phosphate group from any suitable substrate. It occurs in the cell cytoplasm and mitochondrial matrix.
- Oxidative phosphorylation is phosphorylation of ADP at the cost of energy released during oxidation of substrates like NADH+ H+ and FADH2. This occurs on the inner mitochondrial membrane only.
Whenever the cell requires energy, ATP is hydrolysed to ADP and Pi and energy is released, as high energy phosphate bond is broken.
ATP as Energy Currency of Cell :
The term ATP was coined by Karl Lohman in 1929 and ATP cycle was discovered by Lipmann in 1941. Lipmann is called father of ATP cycle.
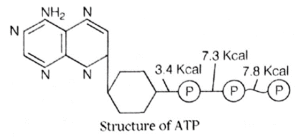
- Chemically, ATP is a triphosphate ester of adenosine ribonucleoside.
- The chemical components include the nitrogenous base-(purine) called adenine and a pentose sugar (ribose) to which three phosphate groups are added.
Adenine + Ribose = Adenosine.
- Adenosine + Phosphate =Adenosine Monophosphate (AMP).
- AMP + Phosphate = Adenosine Diphosphate (ADP).
- ADP + Phosphate = Adenosine Triphosphate (ATP).
- The two terminal phosphate groups are linked by high energy bonds and most of the energy conversions in the cell takes place at ADP and ATP level.
Therefore, ATP is known as universal energy carrier or the energy currency of cells. Hydrolysis of ATP releases 7.28 kcal of energy. ATPase enzyme catalyses the condensation reaction of ATP.
Mechanism of Respiration :
Respiration may takes place by two ways-aerobic and anaerobic.
The respiration which occurs in the presence of oxygen (O2) is called aerobic respiration and the respiration which occurs in the absence of oxygen is called anaerobic respiration.
(I) Anaerobic Respiration :
- It is an enzyme mediated, energy liberating catabolic stepwise process, but there is incomplete breakdown of organic substrates without using oxygen as an oxidant. H2O is not produced as end product in this process.
- Efficiency of anaerobic respiration is very low as out of 686 kcal of energy from a glucose molecule, 14.6 kcal (2ATP = 2 x 7.3 kcal) energy is produced in biologically usable form, per cent of anaerobic respiration efficiency is only 2.12%.
- Process of anaerobic respiration takes place completely in cytoplasm only.
- In microorganisms, the term 'anaerobic respiration' is replaced by 'fermentation' (Cruickshanic; 1897), which is known after the name of its major product, e.g. alcoholic fermentation and lactic acid fermentation.
- Glycolysis is common to both aerobic and anaerobic respiration.
Fermentation :
It is the exclusive mode of respiration in many prokaryotes, several unicellular eukaryotes and moulds.
It is the general term for such processes which extract energy (as ATP), but do not consume oxygen or change the concentration of NAD+ or NADH and is similar to anaerobic respiration.
The two major types of fermentation are as follows
(i) Alcoholic fermentation (common in yeast) : The breakdown of the substrate takes place outside the cell to form ethyl alcohol, CO2 and energy. It is used in brewing industry. In this, the pyruvate is decarboxylated to acetaldehyde. The acetaldehyde is then reduced to NADH + H+ to ethanol.
Sometimes, excess accumulation of ethanol is yeast culture can lead to death of cells.
(ii) Lactic acid fermentation (common in Lactobacillus) : Here, the NADH + H+ produced during glycolysis is reoxidised to NAD+ by donating one proton and two electrons to pyruvic acid which yields lactic acid.
It is used in the production of curd, cheese and yoghurt. It also occurs in muscles of higher organisms during vigorous exercise.
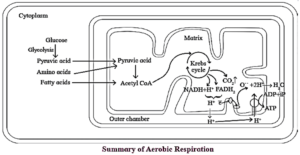
(II) Aerobic Respiration :
It is stepwise catabolic process of complete oxidation of organic food into CO2 and water with oxygen acting as a terminal oxidant, that takes place in cytosol.
It is completed in two pathways, i.e. common pathway and Pentose Phosphate Pathway (PPP).
Common pathway of aerobic respiration consists of three steps
- Glycolysis
- Krebs' cycle
- Electron transport chain or terminal oxidation.
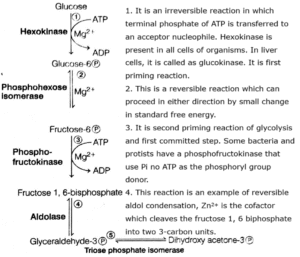
(1) Glycolysis :
- The scheme of glycolysis was given by Gustav Embden, Otto Meyerhof and J Parnas and is often referred as the EMP pathway
- Glycolysis is a process where glucose is broken down into two molecules of pyruvic acid, hence called glycolysis (glucose-breaking).
- It is common to both aerobic and anaerobic respiration.
- Glycolysis (Gr. Glycos-sugar; lysis-splitting), is a stepwise process by which one molecule of glucose (6C) breaks down into two molecules of pyruvic acid (3C). It involves ten steps.
- Glycolysis occurs in the cytoplasm of the cell. During the process, glucose gets partially oxidised.
The net result of glycolysis can be given as follows
Balance sheet of glycolysis :
(i) Reaction : ATP formation by substrate level phosphorylation
| Steps | Products | ATP |
| 1, 3-diphosphoglyceric acid (2 moles) --> 3 phosphoglyceric acid (2 moles) | 2 ATP | 2 ATP |
| Phosphoenolpyruvic acid (2 moles) --> Pyruvic acid (2 moles) | 2 ATP | 2 ATP |
| Total | 4 ATP |
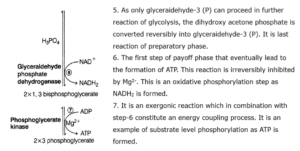
(ii) Reaction : ATP formation by oxidative phosphorylation or ETC
| Steps | Products | ATP |
| Glyceraldehyde 3 phosphate -> 1, 3-bisphosphoglycerate (2 moles) | 2 NADH2 | 6 ATP |
| Total ATP formed | 4 + 6 = 10 ATP |
(iii) Reaction : ATP consumed in glycolysis
| Steps | Products | ATP |
| Glucose (1 mol) ->Glucose 6 phosphate (1 mol) | -1 ATP | -1 ATP |
| Fructose 6 phosphate (1 mol) -> Fructose 1, 6-diphosphate (1 mol) | -1 ATP | -1 ATP |
| Total ATP consumed | 2 ATP |
Net gain ATP = total ATP formed - total ATP consumed = 10 ATP - 2 ATP = 8 ATP
Schematic Representation of EMP Pathway :
Differences between glycolysis and fermentation :
| Glycolysis | Fermentation |
| It is the first step of respiration which occurs without requirement of oxygen and is common to both aerobic and anaerobic mode of respiration. | It is anaerobic respiration which does not require oxygen. |
| It produces pyruvic acid. | It produces different products. The common ones are ethanol, CO2 and lactic acid. |
| It produces two molecules of NADH per glucose molecule. | It generally utilises NADH produced during glycolysis. |
| It forms 2ATP molecules per glucose molecule. | It does not produce ATP. |
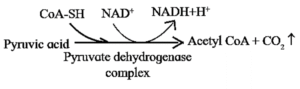
Oxidative Decarboxylation of Pyruvic Acid :
- In the presence of sufficient O2, each 3-carbon-pyruvate molecules (CH3COCOOH) enters in the mitochondrial matrix, where its oxidation is completed by aerobic means.
- This reaction is also called as the transition reaction or link reaction between glycolysis and Krebs' cycle. It can be shown as follows
- Thus, acetyl Co-A acts as a connecting link between glycolysis and citric acid cycle.
- During this process, two molecules of NADH are produced from the metabolism of two molecules of pyruvic acid (produced from one glucose molecule during glycolysis).
Note Pyruvate dehydogenase complex needs thiamine (Vitamin-B1) as a coenzyme. It cannot function in the absence of vitamin-B1.
Hence, thiamine deficiency causes pyruvic acidosis and lactic acidosis.
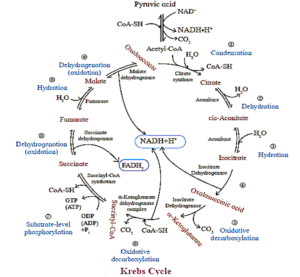
(2) Krebs' Cycle or Tricarboxylic Acid Cycle :
It is also known as citric acid cycle because citric acid (tricarboxylic acid) is the first product of this cycle.
- In eukaryotic organisms, all the reactions of Krebs' cycle takes place in matrix of mitochondria because enzymes of this cycle are present in matrix except succinic dehydrogenase (situated in inner membrane of mitochondria).
- In prokaryotes, the Krebs' cycle occurs in cytoplasm. It is basically a catabolic reaction, as it oxidise acetyl Co-A and organic acid into CO2 and H2
- It acts as a amphibolic pathway because it serves in both catabolic and anabolic processes. It is a series of 8 reactions which occurs in aerobic environment.
The Krebs' cycle discovered by Sir Hans Krebs in 1937, comprises of following reactions or steps in given sequence
- Step 1 : Acetyl Co-A adds its 2-carbon fragment to oxaloacetate, a 4-carbon compound, catalysed by citrate synthase. The unstable bond of acetyl Co-A is broken as oxaloacetate, displaces the coenzyme and attaches to the acetyl group. The product is the 6-carbon citrate.
- Step 2 : A molecule of water is removed and another is added back. The net result is the conversion by using enzyme aconitase citrate to its isomer, isocitrate.
- Step 3 : The substrate loses a CO2 molecule and the remaining 5-carbon compound is oxidised, reducing NAD+ to NADH catalysed by isocitrate dehydrogenase.
- Step 4 : This step is catalysed by a multi-enzyme complex α-keto glutarate dehydrogenase CO2 is lost, the remaining 4-carbon compound is oxidised by the transter of electrons to NAD+ to form NADH and is then attached to coenzyme-A by an unstable bond.
- Step 5 : Substrate level phosphorylation is carried out using succinyl Co-A synthetase. Co-A is displaced by a phosphate group, which is then transferred to GDP to form Guanosine Triphosphate (GTP). GTP is similar to ATP, which is formed when GTP donates a phosphate group to ADP.
- Step 6 : Oxidative step. 2 hydrogen are transferred to FAD+ to form FADH2 by succinyl dehydrogenase
- Step 7 : Bonds in the substrate are rearranged by fumarase by the addition of a water molecule.
- Step 8 : The last oxidative step catalysed by malate dehydrogenase produces another molecule of NADH and regenerates oxaloacetate which accepts a 2-carbon fragment from acetyl Co-A for another turn of the cycle.
The balance sheet of Krebs' cycle, i.e. ATP formed and consumed can be summarised as follows.
Balance sheet of Krebs' cycle :
Reactions : (i) ATP formation by substrate level phosphorylation
| Steps | Products | ATP |
| Succinyl Co-A (2 mol) -> Succinic acid (2 mol) | 2 GTP | 2 ATP |
| Total | 4 ATP |
Reactions : (ii) ATP formation by oxidative phosphorylation or ETC
| Steps | Products | ATP |
| Pyruvic acid (2 mol) -> Acetyl Co-A (2 mol) | 2NADH2 | 6 ATP |
| Isocitric acid (2 mol) -> Oxalosuccinic acid (2 mol) | 2NADH2 | 6 ATP |
| α-ketoglutaric acid (2 mol) -> Succinyl Co-A (2 mol) | 2NADH2 | 6 ATP |
| Succinic acid (2 mol) -> Fumaric acid (2 mol) | 2FADH2 | 4 ATP |
| Malic acid (2 mol) -> Oxaloacetic acid (2 mol) | 2NADH2 | 6 ATP |
| Total | 28 ATP |
Net gain of Krebs' cycle (substrate level phosphorylation + Oxidative phosphorylation) = 2ATP + 28ATP = 30 ATP
Net reaction of respiration (glycolysis and Krebs' cycle) can be given as
Glucose + 4ADP + 4H3PO4 + 10NAD+ + 2FAD+ → 6CO2 + 4ATP + 10NADH + 10H+ + 2FADH2
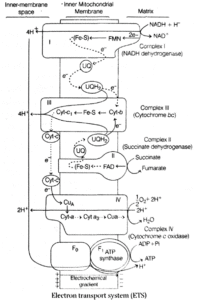
(3) Electron Transport Chain (ETC) :
Electron Transport Chain (ETC) or Respiratory Chain (RC) is present in the inner membrane of mitochondria.
When the electron pass from one carrier to another in electron transport chain, they are coupled to ATP synthase for the production of ATP from ADP and inorganic phosphate (Pi).
The enzymes of inner membrane appear to exist as components of these five complexes.
These complexes are
- Complex I : NADH/NADPH Co-Q reductase or NADH-dehydrogenase.
- Complex II : Succinate Co-Q reductase or succinate dehydrogenase.
- Complex III : Reduced Co-Q (Co - QH2) or cytochrome-c reductase (consists of cyt-b, FeS complex and cyt-c1)
- Complex IV : Cytochrome-c oxidase (comprises of cyt-a and cyt-a3).
- Complex V : ATP synthase complex which has a head piece, stalk and a base piece.
The head piece is F1 (coupling factor 1) and the base piece is F0 (present within mitochondrial membrane and act as a proton channel). Together Fo -F, complex helps in synthesis of ATP from ADP and Pi. This is oxidative phosphorylation.
As transfer of protons is accompained with synthesis of ATP, this process is named as chemiosmosis by Peter Mitchell.
The first four members among these complexes constitute the electron transport system, while the 5th complex is connected with oxidative phosphorylation, i.e. conservation and transfer of energy with ATP synthesis.
A diagrammatic representation of electron flow via various electron carrier complexes is shown in figure.
The correct sequence of electron accepter are cyt-b -> c -> a ->a3.
Oxidation of one molecule of NADH gives rise to 3 molecules of ATP, while FADH2 produces 2 molecules of ATP. However, the number of ATP produced depends upon the physiological conditions and source of respiratory substrate.
Significance of ETS :
- The electron transport system (ETS) or terminal oxidation generates major amount of energy in the form of ATP molecules, 34 ATP molecules out of total 38 ATP molecules are produced through ETS.
- It regenerates oxidized coenzymes such as NAD+ and FAD+ from their reduced forms (NADH+H+ and FADH2) for recycling.
- It also produces water molecules.
- It releases energy in a stepwise manner to prevent damage of cells.
Balance sheet for ATP by aerobic oxidation of 1 glucose molecule
Glycolysis, TCA cycle and electron transport chain link :
- The coenzymes are initially present in the form of NAD+ and FAD+ which latter get reduced to NADH+H+ and FADH+H+ by accepting the hydrogen from organic substrate during glycolysis, link reaction and Kerbs cycle.
- During glycolysis, glucose is oxidised to two molecules of pyruvic acid with net gain 2 molecules of NADH+H+.
- This pyruvic acid undergoes link reaction to form two molecules of acetyl CoA and two molecules of NADH+H+.
- Acetyl CoA, thus formed enters into the Krebs cycle and it gets completely oxidised to CO2 and H2O with a net gain of 6 NADH+H+ and 2 FADH+H+ are formed.
- During ETS, reduced coenzymes are reoxidized to NAD+ and FAD+ with a net gain of 34 ATPs.
- The ATPs thus formed are used during glycolysis.
- The oxidized NAD+ and FAD+ will again accept the hydrogen from organic substrate. Thus, reduced coenzymes are converted back to their oxidized forms by dehydrogenation to keep the process going.
Differences between aerobic respiration and anaerobic respiration :
| Aerobic respiration | Anaerobic respiration |
| Occurs in the presence of oxygen. | Occurs in the absence of oxygen. |
| Requires participation of ETS or respiratory chain. | Does not require ETS. |
| Phase-I reactions completed in cytosol and phase-Il reactions in mitochondria. | Both phase-I and phase-Il reactions completed in cytosol only. |
| Phase-I reactions are anaerobic in nature and phase-Il reactions are aerobic in nature. | Entire process (phase-I + phase-Il reactions) are anaerobic in nature. |
| Involves terminal oxidation and oxidative phosphorylation | Terminal oxidation and oxidative phosphorylation are not involved. |
| NADH2 and FADH2 formed in the process are oxidised through ETS during terminal oxidation. | NADH2 formed during phase-I is utilised for reduction of CH3CHO to form ethyl alcohol in phase-II. |
| Percentage of energy conservation and efficiency of respiration is 40%. | Percentage of energy conservation and efficiency of respiration is 2%. |
| Total ATP made available in aerobic respiration of one C6H12O6 is 38 ATP | Total ATP made available in anaerobic respiration of one C6H12O6 is 2 ATP. |
| Overall chemical equation
C6H12O6+6O2 -> 6CO2+6H2O+38ATP |
Overall chemical equation
C6H12O6 -> 2C2H5OH + 2CO2+2 ATP |
Utility of Stepwise Oxidation :
Both anaerobic and aerobic respiration are conducted in many steps. These stepwise metabolism has several purposes.
- A stepwise release of chemical energy facilitates the utilisation of higher proportion of energy in ATP synthesis.
- Stepwise activities of enzymes controls the rate of the pathway and the energy output according to need of the cell.
- This pathway can be utilised for forming intermediates used in the synthesis of biomolecules.
Amphibolic Pathway :
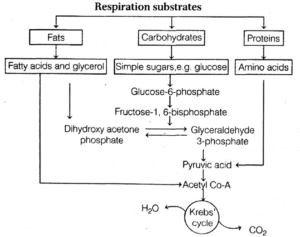
- Carbohydrates are converted to glucose before respiration, while fats are broken down into glycerol and fatty acids.
- Proteins are degraded by proteases, and individual amino acids enter the pathway during the Krebs' cycle or as pyruvate or acetyl Co-A.
- The respiratory pathway is involved in both anabolism and catabolism of fatty acids and proteins, making it an amphibolic pathway rather than a catabolic one.
A general representation of amphibolic pathway is as follows :
Interrelationship among metabolic pathway showing respiration mediated breakdown of different organic molecules to CO2 and H2O.
Respiratory Quotient :
Ratio of volume of CO2 released to the volume of O2 consumed in respiration is called the respiratory quotient (RQ) or respiratory ratio. It depends on the type of respiratory substrate.
R.Q. = \(\frac{volume\,of\,CO_2\,released}{volume\,of\,O_2\,consumed}\)
RQ is measured by Ganong's respirometer.
The RQ for different respiratory substrates :
Carbohydrates (R.Q. is 1) :
When carbohydrates are used as substrate, equal volumes of CO2 and O2 are evolved and consumed respectively, thus its R.Q. is 1.
C6H12O6 + 6O2 → 6CO2 + 6H2O
R.Q. = 6CO2/6O2 = 1.0
Fats (R.Q. is less than 1):
Substrates like fats are poorer in oxygen than carbohydrates. Thus, more oxygen is utilized for its complete oxidation.
2(C51H98O6) + 145O2 → 102CO2 + 98H2O + Energy
R.Q. = CO2/O2 = 102/145 = 0.7
Protein respiration (R.Q. is less than 1) :
- When proteins serve as respiratory substrate, they are first degraded to amino acids.
- Then, amino acids are converted into various intermediates of carbohydrates.
- However, amino acids have low proportion of O2 as compared to carbohydrates.
- Thus, they require more O; during their complete oxidation and value of R.Q. becomes less than 1.
- In case of proteins, the R.Q. is approximately 0.9.
Various substrate and their RQ :
| Substrates | RQ |
| Carbohydrate | 1 |
| Fats | 0.7 |
| Proteins | 0.9 |
| Organic acids | >1 |
| Succulents | 0 |
| Mixed diet | 0.85 |
| Organic acids (oxalic acid) | 4 |
| Anaerobic respiration | Infinity |
Factor Affecting Respiration :
Various factors affecting respiration can be summarised as
- Amount of oxygen Rate of respiration is directly proportional to amount of oxygen available.
- Intensity of light Rate of respiration is directly proportional to intensity of light.
- Temperature The rate of respiration is directly proportional to temperature.
- Dehydration Rate of respiration is inversely proportional to dehydration.
- Minerals Rate of respiration is directly proportional to availability of minerals as some of them play vital role in the process, i.e. acts as catalysts on reaction
- Tissue injury The rate of respiration is directly proportional to tissues injury.
Significance of Respiration
- Respiration provides energy for synthesis of biomolecules.
- It is also a source of energy for cell division, growth, repairs and replacement of worn out parts, movements, locomotion etc.
- Various intermediates of Krebs cycle are used as building blocks for synthesis of other complex compounds.
- Coupled with photosynthesis, it helps to maintain the balance between CO2 and O2 in the atmosphere.
- Anaerobic respiration (fermentation) is used in various industries such as dairies, bakeries, distilleries, leather industries, paper industries etc. It is used in the commercial production of alcohol, organic acids, vitamins, antibiotics etc.
PDF : MHTCET-Biology-Chapter-2-Respiration and Energy Transfer– Notes PDF : MHTCET-Biology-Chapter-2-Respiration and Energy Transfer– MCQ Offer for PDF Set : All Chapters Notes MHT CET Biology (18 PDF) Rs. 145-Buy All Chapters MCQ with Answers- MHT CET Biology (18 PDF) Rs. 155-Buy All Chapters Notes + MCQ with Answers- MHT CET Biology (36 PDF) Rs. 280-Buy
Main Page : - MHTCET-Biology Exam - All chapters notes, MCQ, videos, test, pdf.
Next Chapter : Chapter 1- Biomolecules - Online Notes
Next Chapter : Chapter 3- Human Nutrition - Online Notes
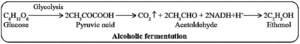



We reply to valid query.