Biomolecules
MHTCET Exam-Biology-Chapter-1
Notes
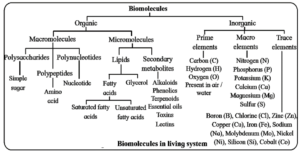
Biomolecules : All the carbon compounds obtained from living tissues are called 'Biomolecules'.
About 99% of living organisms consist of four elements: hydrogen, oxygen, carbon, and nitrogen, which combine in various proportions and ways to form biomolecules. These are defined as the organic molecules present in the living organisms.
- Biomolecules act as building blocks of life and perform important physiological processes.
- They include large molecules (biomacromolecules) like proteins, nucleic acids, polysaccharides, lipids and small molecules (biomicromolecules) such as amino acids, fatty acids, etc.
- Along with organic elements and compounds, living organisms also show presence of inorganic elements and compounds.
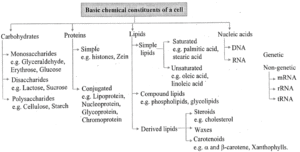
Basic Chemical Constituents of Living Bodies :
- Living bodies cell is made up of over 5000 chemicals.
- The structure and function of different cell constituents are an interplay of their constituent chemicals, their arrangement and properties.
- The average composition of living cell is water (70-90%), proteins (10-15%), carbohydrates (3%), lipids (2%), nucleic acids (5-7%) and ions (1%).
Carbohydrates :
- The word carbohydrates mean 'hydrates of carbon'. They are also called saccharides.
- These are the compounds of carbon, hydrogen and oxygen having hydrogen and oxygen in the same ratio as that of water, i.e. 2 : 1.
- They can be represented by a general molecular formula, [CH2O]n or CnH2nOn.
There are following three types of carbohydrates :
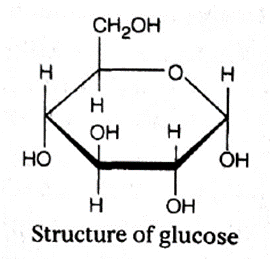
(i) Monosaccharides :
- These are simple carbohydrates that cannot be hydrolysed further into smaller units.
- They consist of a single polyhydroxy aldehyde or ketone unit.
- These are mostly made up of 3-7 carbon atoms.
- Monosaccharides are soluble in water, sparingly soluble in alcohol and insoluble in ether.
- They may exist in different isomeric forms, e.g. glucose, fructose, etc.
- Monosaccharides containing the aldehyde (-C=O) group are classified as aldoses, e.g. glucose, xylose, and those with a ketone (-C=O) group are classified as ketoses, e.g. ribulose, fructose, etc.
Monosaccharides can be further divided on the basis of number of C-atoms as follows
- Trioses (C3H6O3) 3C-atoms asymmetric, e.g. dihydroxy acetone and glyceraldchyde.
- Tetroses (C4H8O4) 4C-atoms, e.g. erythrose and threose.
- Pentoses (C5H10O5) 5C-atoms, e.g. ribose (found in RNA), arabinose (an intermediate metabolite), etc.
- Hexoses (C6H1206) 6C-atom, e.g. glucose (blood sugar), galactose (component of milk sugar), mannose (common in plants), fructose (sweetest of all sugar, found in fruits), etc.
- Heptoses (C7H14O7) 7C-atoms, e.g. sedoheptulose (intermediate in Calvin cycle).
Examples of Monosaccharides :
- Glucose : It is the most important fuel in living cells. Its concentration in the human blood is about 90 mg per 100 mL of blood. The small size and solubility in water of glucose molecules allows them to pass through the cell membrane into the cell. Energy is released when the molecules are metabolised by cellular respiration.
- Galactose : It looks very similar to glucose molecules. They can also exist in a and ß-forms. Galactose react, with glucose to form the dissacharide lactose. However, glu: cose and galactose cannot be easily converted into one another. Galactose cannot play the same role in respiration as glucose.
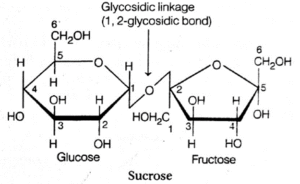
- Fructose : It is the fruit sugar and chemically, it is ketohexose, but it has a five-atom ring rather than a six-atom ring. Fructose reacts with glucose to form the sucrose, a disaccharide.
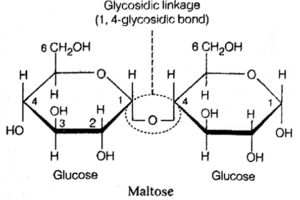
(2) Disaccharides :
- These are formed when two monosacharides react by condensation reaction releasing a water molecule. This process require energy.
- A glycosidic bond forms and holds two monosaccharides limits together.
- Disaccharides are soluble in water, but they cannot pass through the cell membrane by diffusion. They are broken down in the small intestine during digestion.
Examples of disaccharides :
(i) Lactose or Milk Sugar : It is present in milk of mammals and made up of one glucose and one galactose units. It is a reducing sugar. Souring of milk is due to the conversion of lactose to lactic acid.
(ii) Maltose or Malt Sugar : It is named because of its occurrence in malted grain of barley. Mostly found in germinating seeds and tissues, where starch is broken down. It is a reducing sugar and formed by condensation of 2 glucose units.
(iii) Sucrose or Table Sugar : It is also known as cane sugar or invert sugar. In this, fructose occurs in pentagon form, while glucose is in hexagon form. It is a non-reducing sugar.
(3) Polysaccharide :
- These are polymers or chains of monosaccharides (usually more than 9) bound in linear or branched chain pattern.
- In a polysaccharide, an individual monosaccharide is linked by glycosidic bonds to another monosaccharide. This is called polymerisation.
- The properties of a polysaccharide depends on its length, branching, folding and coiling.
Examples of polysaccharides :
- Starch (C6H10O5) : It is a polymer of D-glucopyranose units linked by a-1, 4-glycosidic linkage. It consists of a mixture of amylose (linear, 200-500 glucose units) and amylopectin (branched, more than 1000 glucose units) in 1 : 4 ratio respectively. It is the reserve food material in plants. The test of starch is done with iodine solution. Starch gives blue-black colour with iodine reagent.
- Glycogen : About 5000-15000 glucose units make up glycogen (C6H10Os)n. It is extensively branched and forms the reserve food material in animals hence, also called as animal starch.
- Cellulose : It is a linear polymer of ß-D glucose units connected through ß-1, 4-glycosidic linkage. It is an important structural component of the cell wall of plants.
Biological Significance of Carbohydrates :
- Carbohydrates provide energy for metabolism.
- Glucose is the main substrate for ATP synthesis.
- Lactose, a disaccharide present in the milk provides energy to babies.
- Polysaccharide serves as a structural component of cell membrane, cell wall and reserved food as starch and glycogen.
Lipids :
- These are heterogenous group of compound having one characteristic in common, i.e. their hydrophobic nature.
- They are made up of carbon, hydrogen and oxygen. The count of oxygen atoms in them is always less as compared to the number of carbon atom.
- These are insoluble in water, but are readily soluble in non-polar organic solvent such as chloroform, benzene and ether. The term lipid was given by Bloor. In most of the forms, fatty acids are major constituents of lipids.
Fatty Acids :
These are water insoluble long chain hydrocarbons (4-36 carbon long) with one carboxyl (-COOH) group. These are the simplest constituents of lipids.
The fatty acids are of two types as follows
- Saturated fatty acids These are fatty acids with no double bonds between the carbon atoms of the hydrocarbon chain, e.g. palmitic and stearic acids.
- Unsaturated fatty acids These are fatty acids with one or more double bonds between the carbon atoms of the hydrocarbon chain, e.g. oleic acid and linoleic acid.
Classification of lipids :
(i) Simple Lipids : These are the esters of fatty acids with alcohol. These are of two types, i.e.
- Triglycerides (Neutral fats) : Neutral fats such as butter and vegetables oils are mostly triglycerides. Each has three fatty acids linked to a glycerol (glycerine or trihydroxy propane). In fats, when all three fatty acids are similar, they are called pure fats and when these fatty acids are dissimilar, they termed as mixed fat.
- Waxes : These are long chain fatty acid linked to long chain of alcohol or carbon ring. All waxes have firm consistency and repel water. In plants, they cover the surface of leaf and other aerial surfaces to avoid excess transpiration. In animals, cutaneous glands secretes wax (lanolin), for forming a protective water insoluble coating on animal fur.
(ii) Compound or Conjugated Lipids :
- These are esters of fatty acids with alcohol, but contain some other substances also, e.g. phospholipids, glycolipids, lipoproteins, etc.
- They contain a molecule of glycerol, two molecules of fatty acids and a phosphate group or simple sugar.
- Phospholipids have both hydrophilic polar groups (phosphate and nitrogenous group) and hydrophobic non-polar groups (hydrocarbon chains of fatty acids).
- Phospholipids contribute in the formation of cell membrane.
- Glycolipids contain glycerol, fatty acids, simple sugars such as galactose. They are also called cerebrosides as they are largely found in the brain white matter and nerve mylin sheath.
(iii) Derived Lipids :
- These are obtained by hydrolysis of simple and compound lipids. Sterols is a category of derived lipids composed of fused hydrocarbon rings (steroid nucleus) and a long hydrocarbon side chain,
- g. cholesterol (a widely distributed molecule in all cells of the animal body, particularly in nervous tissue.
- Cholesterol exists either free or as cholesterol ester.
- Adrenocorticoids, sex hormones (progesterone, testosterone) and vitamin D are synthesized from cholesterol.
- Cholesterol is not found in plants.
- Sterols exist phytosterols in plants.
- Yam Plant (Dioscorea) produces a steroid compound called diosgenin. It is used in the manufacture of antifertility pills. i.e. birth control pills.
Proteins :
The term 'protein' was first coined by Berzelius (in 1837) and Mulder (1838). Proteins are involved in structural support, storage, transport, signalling, movement, etc. in living organisms. These are the linear, unbranched polymers of amino acids.
General Structure of Protein :
- In a long chain of amino acids forming a protein, the amino group (-NH2) of one amino acid is linked to the carboxyl group (-COOH) of the other amino acid. The two amino acids are condensed by removal of a water molecule to form peptide linkage.
- The residue formed is called dipeptide, of three residues as tripeptide and of many residues as polypeptide.
- During elongation of polypeptide chain, a new amino acid can be added at either end due to free amino or carboxyl group.
- The linear sequence of amino acids in polypeptide chain of a protein forms the primary structure of a protein.
- There are two types of secondary structure of protein: a-helix and ß-pleated sheets.
- The polypeptide chain is arranged in a spiral helix. These spiral helices are of two types: ∝-helix (right handed) and β-helix (left handed). This spiral configuration is held together by hydrogen bonds.
- In tertiary structure the peptide chains are much looped, twisted and folded back on themselves due to formation of disulphide bonds. Such loops and bends give the protein a tertiary structure. E.g. Myoglobin, enzymes.
- When a protein has more than two polypeptide subunits their arrangement in space is called quaternary structure. E.g. Haemoglobin.
Classification of Proteins on the Basis of Structure :
Proteins are classified on the basis of increasing complexity in their structure. These can be of following types
- Simple proteins : Those proteins, which are made up of amino acid only come under this category. This simply means that on hydrolysis they yield only amino acids, e.g. albumins, globulins, glutelins, prolamins, etc.
- Conjugated proteins : These proteins contain some non-proteinaceous group or substance, i.e. prosthetic group in them. It simply means on hydrolysis, they yield some other substances along with amino acids, e.g. histones, serum, lipoproteins, etc.
- Derived proteins : When proteins are hydrolysed by acids, alkalis or enzymes, the degradation products obtained from them are called as derived proteins.
Classification of Proteins on the Basis of Function :
Proteins can also be classified on the basis of their functions, which are as follows
- Enzymatic, e.g. pepsin, trypsin, RuBisCo (most abundant protein in the biosphere), etc.
- Regulatory, e.g. insulin, growth hormone, oxytocin, etc.
- Transporting, e.g. haemoglobin, serum, albumin, etc.
- Storage, e.g. casein, ferritin, glutelins, globulins, etc.
- Contractile and motile, e.g. actin, myosin, tubulin, etc.
- Structural, e.g. collagen, a-keratin, elastin, chondrin, ossein, etc.
- Protective, e.g. immunoglobulin (antibody), thrombin, fibrinogen, etc.
- Exotic, e.g. monellin, glue proteins, etc.
Nucleic Acids :
- They constitute the genetic material of all living organism.
- The two most important nucleic acids present in living cell are Deoxyribonucleic Acid (DNA) and Ribonucleic Acid (RNA). DNA and RNA were discovered by Friedrich
- Miescher in 1969.
- These are macromolecules, made up of polymeric units called as nucleotides.
A nucleotide is composed of following three molecules
(i) Phosphoric acid, i.e. H3PO4 with 3 reactive –OH groups. Out of these 2 are involved in forming sugar phosphate backbone with the help of phosphodiester
linkage (-C-O-P-O-C-)
(ii) Pentose sugar, i.e. the carbohydrate (monosaccharide) with 5 C-atoms. This may be deoxyribose in DNA or ribose in RNA.
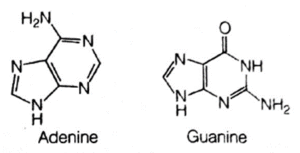
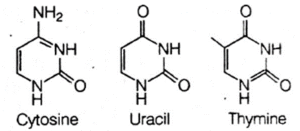
(iii) Nitrogenous bases, i.e. the heterocyclic organic bases, that contain nitrogen in them. There are five different bases, which are categorised mto two types, i.e. purines and pyrimidines.
(a) Purines are double-ring bases. The two purines are adenine and guanine.
(b) Pyrimidines are single ring bases, which include cytosine, thymine and uracil.
The sugar molecule and nitrogenous base together form the nucleoside. It further join with phosphate group to form nucleotide.
Nucleoside + Phosphate group = Nucleotide
DNA :
- It is the main genetic material of living beings.
- In prokaryotes, it is present without any association with proteins.
- However, in eukaryotes it is present with proteins like histones and protamines.
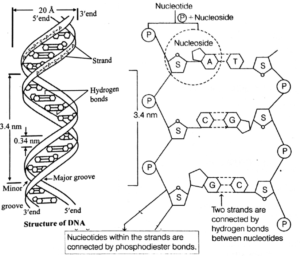
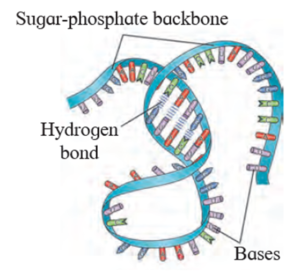
Structure of DNA :
The most widely accepted structural model of DNA is the double helical model of Watson, Crick and Wilkins (1953).
According to the model proposed by Watson and Crick
- The DNA molecule consists of two helically twisted antiparallel strands connected together via base pairs.
- The twisting or coiling of strands results into the formation of deep (major) and shallow (minor) grooves.
- Each strand of DNA consists of alternate molecules of deoxyribose sugar and phosphate groups. The linkage between the sugar and phosphate molecule is called phosphodiester linkage.
- The two strands are interwined in a clockwise direction.
- DNA has a uniform thickness of 2nm (20A) Its pitch or 1 turn is about 0.34 nm.
RNA :
The other nucleic acid present in the cell is RNA, i.e. ribonucleic acid. It is found predominantly in cytoplasm.
It is mostly present in single stranded form though some viruses like retrovirus and wound tumour virus have double-stranded RNA.
The single strand RNA is folded upon itself either entirely or in certain regions. All normal RNA chains begin with adenine or guanine.
Types of RNA
The RNA is of following three types
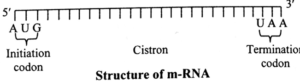
(i) mRNA (messenger RNA) or template RNA : The mRNA carries message (information) from DNA about the sequence of particular amino acids to be joined to form a polypeptide.
It makes only about 2-5% of total cellular RNA and has very short life. mRNA consists of a 5' methylated cap region, is coding region and poly-A tail region.
Coding region separated with cap region by leader region and with tail by trailer region.
There are some differences in mRNA of prokaryotes and eukaryotes.
- Prokaryotic mRNA They are polycistronic (having coding sequences for multiple polypeptide). Their translation begins during transcription only. They are short lived and hardly undergo any processing, hence they do not possess a poly-A tail at 3' end.
- Eukaryotic mRNA They are monocistronic (having coding sequence only for one polypeptide). Their translation begins only when the transcription has been completed.
Thus, it is more stable than the prokaryotic mRNA. The transcribed RNA (hnRNA) undergoes processing (capping, methylation and polyadenylation) to form the mRNA molecule.
(ii) rRNA (Ribosomal RNA) : This RNA is the basic component of ribosomes and forms about (70-80%) of total cellular RNA. It forms a highly coiled structure.
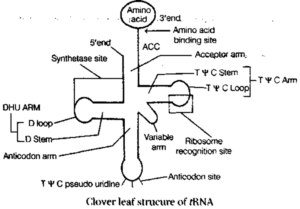
(iii) tRNA (Transfer RNA) : tRNA has many varieties, each carries a specific amino acid from the amino acid pool to mRNA on the ribosome to form a polypeptide. It forms 15% of total cellular RNA.
Its secondary structure also called as clover leaf structure was worked out by Holley et.al. (1965) and is depicted as below.
The structure contains five arms each with a loop and a stem. These are as follows
- Acceptor stem : The arm without loop, contains 7 base pairs and 4 unpaired bases.
- D-arm : The arm with 3-4 base pairs and 7-11 nitrogenous bases, which are unpaired.
- Anticodon arm : The arm with 5 base pairs and 7 unpaired nitrogenous bases.
- Variable arm : The arm which vary, i.e. it may or may not contain stem.
- T Y C arm : The arm with 5 base pairs and 7 unpaired nitrogenous bases.
Enzymes :
- Enzymes are proteinaceous substances that alter the rate of chemical reactions in biological systems without altering themselves.
- The term was coined by Friedrich Wilhelm Kuhne in 1878 while working on fermentation.
- In 1987, German chemist Eduard Buchner isolated the enzyme zymase from yeast cells and demonstrated its ability to ferment sugar, earning him the Nobel Prize.
- Later, JB Sumner (1926) prepared a pure crystalline form of urease enzyme from jack beans and suggested that enzymes are mostly proteins.
Classification of Enzymes :
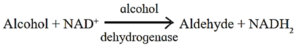
(i) Oxidoreductases : These are enzymes catalyzing oxidation and reduction reactions by the transfer of hydrogen and/or oxygen.
e.g. alcohol dehydrogenase
- Oxidases : Transfers H2 molecule from a molecule to E.g. Cytochrome oxidase (involve in ETS).
- Dehydrogenases : Transfers H2 molecule from a molecule to a coenzyme such as NAD+.g. Succinic dehydrogenase.
(ii) Transferases : These enzymes catalyse the transfer of certain groups between two molecules.
e.g. glucokinase, Transphosphorylases, transaminases, kinases, transpeptidases, etc.
(iii) Hydrolases: These are enzymes catalyse hydrolytic reactions. This class includes amylases, proteases, lipases etc.
- Sucrase, Digestive enzymes like lipases, amylases, esterases, phosphatases, carbohydrases, proteases belong to this group.
Sucrose \(\underrightarrow{Sucrase}\) Glucose + Fructose + H2O
(iv) Isomerases : These enzymes catalyze structural rearrangements within a molecule. Their nomenclature is based on the type of isomerism. Thus these enzymes are identified as racemases, epimerases, isomerases, mutases,
e.g. xylose isomerase. Epimerase, racemase, phosphohexose isomerase (phosphoglucomutase).
Glu-6-Phosphate \(\underrightarrow{Isomerase}\) Fructose-6-Phosphate
(v) Ligases or Synthetases : These are the enzymes which catalyse the covalent linkage of the molecules utilizing the energy obtained from hydrolysis of an energy-rich compound like ATP, GTP
e.g. glutathione synthetase, Pyruvate carboxylase, DNA ligases (repair breaks in DNA molecule), amino acyl synthetase (activate tRNA by attaching amino acid at 3'end).
Pyruvate + CO2 + ATP \(\underrightarrow{Pyruvate\,Carboxylase}\) Oxaloacetate + ADP + Pi
Properties of Enzymes :
- Enzymes do not get consumed in a reaction, rather they are involved in increasing the rate of chemical reaction.
- They are colloidal in nature.
- They are highly specific, i.e. an enzyme generally catalyses a specific reaction.
- They are thermolabile (heat sensitive), 25°-35℃ is considered the best or optimum temperature for their functioning. Their activity stops at 0 ℃ and above 80 ℃.
- The reactions controlled by enzymes are mostly reversible.
- Change in pH may influence the working of an enzyme. An optimum pH of 6-7.05 is required for an enzyme to show its maximum activity.
- Efficiency of an enzyme is expressed in terms of its turn over number. It is the number of moles of substance transformed into products per mole of enzyme per second.
- An enzyme remains unchanged at the end of the reaction.
Chemical Nature of Enzymes :
All enzymes are globular proteins with the exception of recently discovered RNA enzymes.
Some enzymes may additionally contain a non-protein group. There are two types of enzymes on the basis of composition
(1) Simple enzyme : Made up of only peptide chain thus, possess only protein part, e.g. urease, amylase, pepsin.
(2) Conjugate enzyme : Composed of protein and non-protein part.
- Apoenzyme is a large, specific protein part of an enzyme that is thermodynamically stable and does not participate in group transfer.
- Co-factor is a non-protein part of an enzyme, is small, heat stable, and involved in group transfer, allowing it to be used in various reactions.
(i) Prosthetic group : Organic compound which bind covalently to a moiecule (apoenzyme), e.g. haem, etc.
(ii) Coenzyme : Organic compound, which binds to apoenzyme transiently. Vitamins are the essential component of co-enzyme.
(iii) Metal ions : They form coordinate bond with the side chains of enzyme
and substrate, e.g. Zn is cofactor for proteolytic enzyme, carboxypeptidase
- NAD+ and NADP+ are coenzyme of vitamin-B3 (niacin)
- FAD+ and FMN are coenzyme of vitamin-B2 (riboflavin)
Nomenclature of Enzymes :
- Enzymes are named by adding the suffix- 'ase' to the name of the substrate on which they act e.g. protease, sucrase, nuclease etc. which break up proteins, sucrose and nucleic acids respectively.
- The enzymes can be named according to the type of function they perform. For e.g., dehydrogenase remove hydrogen, carboxylase add CO; decarboxylases remove CO2, oxidases helping in oxidation.
- Some enzymes are named according to the source from which they are obtained. For e.g., papain from papaya, bromelain from the member of Bromeliaceae family, pineapple.
- According to international code of enzyme nomenclature, the name of each enzyme ends with an -ase and consists of double name.
- The first name indicates the nature of substrate upon which the enzyme acts and the second name indicates the reaction catalyzed. For e.g., pyruvic decarboxylase catalyses the removal of CO2 from the substrate pyruvic acid.
- Similarly, the enzyme glutamate pyruvate transaminase catalyses the transfer of an amino group from the substrate glutamate to another substrate pyruvate.
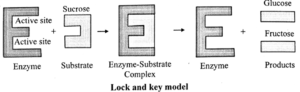
Mechanism of Enzyme Action : The basic mechanism of enzyme action start when an enzyme acts upon a substrate, it forms an enzyme-substrate complex.
Subsequently, this complex decomposes the substrate, undergoes chemical change and the enzyme is regenerat afterwards.
E + S ⇌ ES -> EP ⇌ E+P
Following two models has been put forth to explain the formation of ES complex:
(i) Lock and key model : Proposed by Emil Fisher in 1894. He states that both the components, i.e. enzyme and substrate have strictly complementary structure.
- This model explains the specific action of an enzyme with a single substrate.
- In this model, lock is the enzyme and key is the substrate.
- The correctly sized key (substrate) fits into the key hole (active site) of the lock (enzyme).
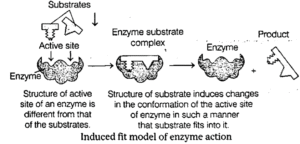
(ii) Induced fit model : Proposed by D Koshland in 1959. According to this, when enzyme bind to substrate, the change in shape of active sites of enzyme takes place.
- It is the more accepted model to understand mode of action of enzyme.
- The induced fit model shows that enzymes are rather flexible structures in which the active site continually reshapes by its interactions with the substrate until the time the substrate is completely bound to it.
- It is also the point at which the final form and shape of the enzyme is determined.
Factors Affecting Enzyme Activity :
The activity of an enzyme can be affected by a change in the conditions which can alter the tertiary structure of the protein.
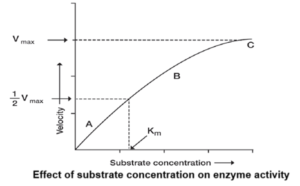
- Substrate concentration : Enzyme activity increase with increase in concentration of the substrate to a maximum and then it levels off.
- Enzyme concentration : In general, the rate of reaction will increase with increasing enzyme concentration, due to availability of more active sites for reaction.
- Temperature and pH : In most of the enzymatic reactions, rise of 10 ℃ in the temperature doubles the rate of reaction between 5-40 ℃. Enzymes are denatured (secondary and above level . structures degraded) at higher temperature due to their proteinaceous nature and rate of reaction drops.
- Redox potential : Enzymes are sensitive to redox potential of the cell. Many enzymes are affected by redox potential due to the presence of oxidisable SH-group.
Michaelis-Menten Concept :
A basic theory of enzyme action was proposed by Michaelis and Menten (1913). They gave an equation to describe how the reaction relatively varies with substrate concentration.
Hence, V0 = \(\frac{V_{max}×[S]}{K_m×[S]}\)
Where,
Km = Michaelis-Menten constant, i.e. the substrate concentration to produce half maximum velocity.
V0 = Velocity of reaction,
Vmax = Maximum velocity of reaction
[S] = Substrate concentration
Enzyme Inhibition :
Reduction or stoppage of enzyme activity due to certain adverse conditions or chemicals is called enzyme inhibition and the chemicals which interferes or inhibits
the process are called inhibitor.
Enzyme inhibition can be of following types
- Competitive inhibition : It is a reversible process due to substrate or enzyme analogue in which Km increases, but Vmax remains the same.
- Non-competitive inhibition : In this, inhibitor forms a complex with enzyme other than the active site and Vmax
- Feedback inhibition : Where the end product or intermediates functions as temporary inhibitor, which combines with a regulatory site (also known as allosteric site) of the enzyme and thus, functions as negative modulator. This is also called allosteric modulation.
Concept of Metabolism :
- Each cell contains thousands of organic compounds. These compounds or biomolecules are present in living organisms in various concentrations.
- Turn over of biomolecules is one of the greatest discoveries. It is the phenomenon in which biomolecules change constantly into some other biomolecules or made from some other biomolecules.
- All these transfer of one biomolecule into other occur due to chemical reaction, which continuously take place in an organism. The chemical reactions together are called metabolism.
Metabolic pathways in living organisms are divided into two main types
- Anabolic Pathways : These include the formation of complex structure from simple ones, e.g. formation of cholesterol from acetic acid, protein synthesis, etc. These are energy consuming pathways.
- Catabolic Pathways : These include the formation of simpler structures, i.e. the breakage of complex structures into simpler ones, e.g. conversion of glucose into lactic acid in skeletal muscles. These are energy releasing pathways.
Metabolic Pool :
- It is the reservoir of biomolecules in the cell on which enzymes can act to produce useful products as per the need of the cell.
- The concept of metabolic pool is significant in cell biology because it allows one type of molecule to change into another type, e.g. carbohydrates can be converted to fats and vice-versa.
A large number of organic biomolecules are present in the cells which are used in various metabolic reactions of cell. Hence, these compounds are called metabolites. These are divided into two types
- Primary metabolites : These are metabolites which are found in animal tissues. Their functions are easily indentifiable. They play specific known roles in the normal physiological processes, e.g. amino acids, carbohydrates, proteins, nitrogen bases, nucleic acids, etc.
- Secondary metabolites : These are metabolites which are generally found in plant, fungal and microbial cells as a byproduct of main metabolic reactions. Their functions are not identifiable in host organism and are not yet understood, e.g. alkaloids, flavonoids, rubber, essential oils, antibiotics, coloured pigments, scents, gums, spices.
Secondary metabolites can be pigments (carotenoids, anthocyanins), alkaloids (morphine, codeine), terpenoides (Monoterpenes Diterpenes), toxins (Abrin, Ricin), drugs (vinblastin, curcumin), Lectins (concanavalin A), essential oils (lemon grass oil), polymeric substances (rubber, gums, cellulose), scents, spices, antibiotics etc.
The functions of all secondary metabolites are not known, but are useful to mankind and have ecological importance.
Both primary and secondary metabolites serve the following functions
- Many of them are used for welfare of human beings, e.g. rubber, drugs, spices, scents, pigments.
- Some have ecological importance, e.g. cellulose
Importance of secondary metabolites :
- Drugs developed from secondary metabolites have been used to treat infectious diseases, cancer, hypertension and inflammation.
- Morphine, the first alkaloid isolated from Papaver somniferum is used as pain reliver and cough suppressant.
- Secondary metabolites like alkaloids, nicotine, cocaine and the terpenes, cannabinol are widely used for recreation and stimulation.
- Flavours of secondary metabolites improve our food preferences.
- Tannins are added to wines and chocolate for improving astringency.
- Since most secondary metabolites have antibiotic property, they are also used as food preservatives.
- Glucosinolates is a secondary metabolite which is naturally present in cabbage imparts a characteristic flavour and aroma because of nitrogen and Sulphur containing chemicals. It also offers protection to these plants from many pests.
PDF : MHTCET-Biology-Chapter-1-Biomolecules– Notes PDF : MHTCET-Biology-Chapter-1-Biomolecules– MCQ Offer for PDF Set : All Chapters Notes MHT CET Biology (18 PDF) Rs. 145-Buy All Chapters MCQ with Answers- MHT CET Biology (18 PDF) Rs. 155-Buy All Chapters Notes + MCQ with Answers- MHT CET Biology (36 PDF) Rs. 280-Buy
Main Page : - MHTCET-Biology Exam - All chapters notes, MCQ, videos, test, pdf.
Next Chapter : Chapter 2- Respiration and Energy Transfer - Online Notes
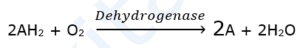
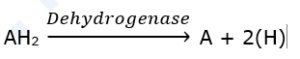
We reply to valid query.