Atoms and Molecules
NCERT-Class-9-Science-Chapter-3
Notes
|
Topics to be learn :
|
Laws of Chemical Combination :
Chemical combination laws are crucial laws that dictate the reactions between elements to form compounds or products.
Antoine L. Lavoisier established two significant laws of chemical combination, which laid the foundation of chemical sciences.
(i) Law of conservation of mass :
It states that, 'mass can neither be created nor destroyed during a chemical reaction.' This means that in any chemical reaction, the total mass of the reactants is equal to the total mass of the products and there is no change in mass during the chemical reaction.
Example : If 6.0 g of sodium carbonate reacts with 15 g of hydrochloric acid, it results in the formation of 3.75 g of carbon dioxide and 17.25 g of sodium chloride solution. Show that these results are in accordance with the law of conservation of mass.
Answer :
Sodium carbonate (6.0 g) + Hydrochloric acid (15.0 g) → Carbon dioxide (3.75 g) + Sodium chloride (17.25 g)
Here, total mass of reactants = 6.0 + 15 = 21 g
Total mass of products = 3.75 + 17.25 = 21 g
Since, the reactants and products have the same mass, this means that there was no loss or gain of mass after the reaction. Hence, it proves the law of conservation of mass.
(ii) Law of constant proportions :
This law was stated by Proust according to which in a chemical substance (or compound), the elements are always present in definite proportions (or ratio) by mass.
Examples :
- In a compound such as water, the ratio of the mass of hydrogen to the mass of oxygen is always 1 : 8, whatever the source of water. Thus, if 9 g of water is decomposed, 1 g of hydrogen and 8 g of oxygen are always obtained.
- In carbon dioxide (CO2 ) always contains carbon and oxygen in the ratio of 3 : 8. If a sample of CO2 contains 36 g of carbon, then it is compulsory that the sample has 96 g of oxygen.
This is calculated as , \(\frac{3}{8}=\frac{36}{x}\) ∴ x = \(\frac{36×8}{3}\) = 96
- Similarly, In ammonia, nitrogen and hydrogen are always present in the ratio 14:3 by mass, whatever the method or the source from which it is obtained.
Scientists were tasked with providing accurate explanations for these laws.
Dalton’s atomic theory provided an explanation for the law of conservation of mass and the law of definite proportions.
Explanation of Laws of Chemical Combination : Dalton's Atomic Theory :
This theory provided an explanation for both the law of chemical combination. According to this theory, all matter whether an element, a compound or a mixture, is composed of small particles called atoms.
The main postulates of Dalton's atomic theory is as follows
- Every matter is made up of very small particles called atoms.
- Atoms are indivisible particles, which can neither be created nor be destroyed in a chemical reaction.
- Atoms of a given element are identical in mass as well as in chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative number and kinds of atoms are constant in a given compound.
What is an Atom?
- Atoms are very small, they are smaller than anything that we can imagine or compare with.
- These are the smallest particles of an element which may or may not have independent existence but take part in chemical reaction. These are the building blocks of all matter.
- The atoms of hydrogen, oxygen, nitrogen, etc., are not capable of independent existence whereas atoms of helium, neon etc., are capable of existing independently
Size of Atoms :
Atoms are very small and their radius is measured in nanometres. (1m =109 nm)
Hydrogen atom is the smallest atom and its radius is 0.1 nm.
What are the modern day symbols of atoms of different elements?
In chemistry, an element is represented by a symbol. Using an element's symbol instead of writing the entire term is easier. Dalton was the first scientist who introduced the symbols for representing elements in a very specific sense.

As Dalton's symbol for elements were difficult to draw and inconvenient to use, modern symbols for the elements were introduced by J J Berzilius.
He suggested that the symbols of elements be made from one or two letters of the name of the element.
In the beginning, the names of elements were derived from the name of the place where they were found for the first time.
Now a days, IUPAC (International Union of Pure and Applied Chemistry) approves the names and symbols of the elements. The first letter of a symbol is always written in capital letter and the second letter in small letter. e.g. Chlorine (Cl), zinc (Zn) and aluminium (Al).
Symbols of some elements have been taken from their names in different languages such as Latin, German, Greek. For example, the symbol of iron is Fe from its Latin name ferrum, sodium is Na from natrium, potassium is K from kalium. Therefore, each element has a name and a unique chemical symbol.
Symbols for some elements :
| Element | Symbol | Element | Symbol | Element | Symbol |
| Aluminium | Al | Copper | Cu | Nitrogen | N |
| Argon | Ar | Fluorine | F | Oxygen | O |
| Barium | Ba | Gold | Au | Phosphorus | P |
| Boron | B | Hydrogen | H | Potassium | K |
| Bromine | Br | Iodine | I | Silicon | Si |
| Calcium | Ca | Iron | Fe | Silver | Ag |
| Carbon | C | Lead | Pb | Sodium | Na |
| Chlorine | Cl | Magnesium | Mg | Sulphur | S |
| Chromium | Cr | Mercury | Hg | Uranium | U |
| Cobalt | Co | Neon | Ne | Zinc | Zn |
Atomic mass :
According to Dalton's, each element has a characteristic atomic mass. But determining the mass of an individual atom was a relatively difficult task due to its very small size.
Hence, their relative atomic masses were determined by using the laws of chemical combinations and the compounds formed.
For this purpose, initially 1/16 of the mass of an atom of naturally occurring oxygen was taken as standard unit because of the following two reasons:
- Oxygen reacted with a large number of elements and formed compounds.
- This atomic mass unit gave masses of most of the elements as whole numbers.
However in 1961 carbon 12 isotope was chosen as standard reference for measuring atomic masses universally. The relative atomic masses of all elements have been found with respect to an atom of carbon-12.
- Atomic Mass Unit : It is defined as the mass unit equal to exactly 1/12th of the mass of one atom of C-12 isotope. Earlier, it was abbreviated as amu but according to latest recommendations of IUPAC, it is now written as 'u'- unified mass.
- Relative Atomic Mass : It is defined as the number of times a given atom is heavier than 1/12th of mass of 1 atom of carbon-12 (C-12) or it is the average mass of the atom as compared to 1/12th the mass of one carbon-12 atom.
Atomic masses of a few elements :
| Element | Atomic Mass (u) |
| Hydrogen | 1 |
| Carbon | 12 |
| Nitrogen | 14 |
| Oxygen | 16 |
| Sodium | 23 |
| Magnesium | 24 |
| Sulphur | 32 |
| Chlorine | 35.5 |
| Calcium | 40 |
How do atoms exist?
Atoms of most elements are not able to exist independently. Atoms form molecules and ions. These molecules or ions aggregate in large numbers to form the matter that we can see, feel or touch.
What is a Molecule?
The smallest particle of an element or compound which is capable of independent existence and shows all the properties of that substance is called a molecule.
In general, molecule is a group of two or more atoms that are chemically bonded together. Atoms of the same element or of different elements can join together to form molecules.
Molecules can be divided into two categories:
(1) Molecules of elements : The molecules of an element contains same type of atoms.
- Molecules of many elements are made up of only one atom of that element. e.g. Noble gases like argon (Ar), helium (He) etc.
- The molecules of most of the non-metals are made up of more than one atom. e.g. A molecule of oxygen (O2) consists of two atoms of oxygen, ozone (O3) consists of three atoms of oxygen.
Atomicity : It is defined as the number of atoms present in a molecule.
On the basis of atomicity, molecules can be classified as:
- Monoatomic molecules : They consist of only one atom. e.g. He, Ne, Ar, Xe, Fe, Al etc.
- Diatomic molecules They consist of two atoms. e.g. H2,O2, N2, I2, Br2, Cl2
- Triatomic molecules They consist of three atoms. e.g. O3
- Tetra-atomic molecules They consist of four atoms. e.g. P4
- Polyatomic molecules They consist of more than four atoms. e.g.S8(octa-atomic) etc.
(2) Molecules of compounds : Atoms of different elements join together in definite proportions to form molecules of compounds.
Molecules of some compounds :
| Compound | Combining Elements | Ratio by Mass |
| Water ( H2O) | Hydrogen and oxygen | 1 : 8 |
| Ammonia (NH3) | Nitrogen and hydrogen | 14 : 3 |
| Carbon dioxide ( CO2) | Carbon and oxygen | 3 : 8 |
| Sulphuric acid ( H2SO4) | Hydrogen, sulphur and oxygen | 1 : 16 : 32 |
Prediction of Number of Atoms from Mass Ratio :
In order to predict the number of atoms from mass ratio, divide the given mass of each element by the atomic mass of the element and calculate the simplest ratio between the obtained moles, e.g. we know that mass ratio of nitrogen and hydrogen in ammonia molecule is 14 : 3.
The number of atoms of nitrogen and hydrogen present in the molecule of ammonia can be calculated as,
| Element | Ratio by Mass (x) | Atomic Mass
(y) |
(x/y) | Simplest Ratio |
| N | 14 | 14 | 14/14 = 1 | 1 |
| H | 3 | 1 | 3/1 = 3 | 3 |
Thus, in ammonia molecule, one N and three H-atoms are present hence, the formula of ammonia is NH3.
What is an ion?
Compounds composed of metals and non-metals contain charged species. These charged species are called ions.
These are of two types, i.e. anions and cations.
(i) Cations : The positively charged ions are known as cations, e.g. Na+, K+, Ca2+, Al3+ etc. These are formed when elements loses electrons. Usually, metals form cations.
(ii) Anions : The negatively charged ions are known as anions, e.g. Cl−, Br−, O2−, N3− etc. These are formed when elements gain electrons. Usually, non-metals form anions.
(iii) Polyatomic lon : A group of atoms carrying charge and act as a single entity
is known as a polyatomic ion. It carries a fixed charge. e.g. NO3− (nitrate ion), CO32− (carbonate ion) and SO42− (sulphate ion) etc.
Some lonic Compounds :
| Ionic Compounds | Constituting Elements | Ratio by Mass |
| Calcium oxide | Calcium and oxygen | 5 : 2 |
| Magnesium sulphide | Magnesium and sulphur | 3 : 4 |
| Sodium chloride | Sodium and chlorine | 23 : 35.5 |
Ionic compounds are neutral compounds formed by cations and anions, e.g. sodium chloride or common salt (NaCl) consists of a positively charged sodium ion (Na+ cation) and negatively charged chloride ion (Cl− anion).
Writing Chemical Formulae :
Valency : The combining power (or capacity) of an element is called its valency.
It can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. The valency of an ion is equal to the charge on the ion.
Names and symbols of some ions :
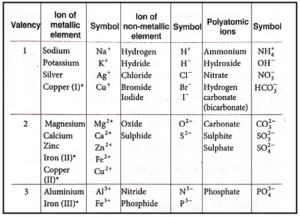
Note : Some elements show more than one valency. Here, the Roman numeral written in brackets shows their valency.
Writing Chemical Formulae : The shortest way to represent a compound with the help of symbols and valency of elements is known as chemical formula.
Chemical formula of a compound shows its constituent elements and the number of atoms of each combining element.
In ionic compounds, the charge on each ion is used to determine the chemical formula of a compound.
There are some rules for writing the chemical formula:
- The valencies or charges on the ion must be balanced.
- When a compound consists of a metal and a non-metal, the symbol of the metal is written first and on the left, whereas non-metal is written on the right. e.g. calcium oxide (CaO), sodium chloride (NaCl), iron sulphide (FeS), copper oxide (CuO) etc., where oxygen, chlorine, sulphur are non-metals and are written on the right, whereas calcium, sodium, iron and copper are metals and are written on left,
- When compound is formed with polyatomic ions, the ion is enclosed in a bracket before writing the number to indicate the ratio. e.g. Ca(OH)2. In case if the number of polyatomic ion is one, the bracket is not required. e.g. NaOH.
Formulae of simple compounds :
Here, rules for writing the chemical formula for simple compounds are given:
- write the symbols of constituent elements and their charge.
- write the symbol of cation first followed by the symbol of anion.
- then criss-cross their charges or valencies to get the formula.
- the positive and negative charges must balance each other and the overall structure must be neutral.
Note : The simplest compounds made up of two different elements are called binary compounds.
Examples :
(i) Aluminium oxide
| Symbol | Al | O |
| Charge | 3+ | 2- |
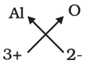
Formula : Al2O3
(ii) Hydrogen sulphide
| Symbol | H | S |
| Charge | 1+ | 2- |
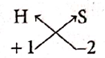
Formula : H2S
Note : When the subscript is number 1, then it does not need to write.
(iii) Carbon tetrachloride
| Symbol | C | Cl |
| Charge | +4 | -1 |
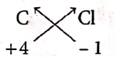
Formula : CCl4
(iv) Calcium Oxide
| Symbol | Ca | O |
| Charge | +2 | -2 |

Formula : Ca2O2 or CaO
Note : When the valency of both elements are numerically equal, the subscripts are not written.
(v) Aluminium Hydroxide
| Symbol | Al | OH |
| Charge | +3 | -1 |
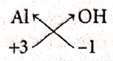
Formula : Al(OH)3
(vi) Ammonium Sulphate
| Symbol | NH4 | SO4 |
| Charge | +1 | -2 |
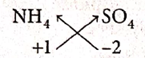
Formula : (NH4)2SO4
Note : We use brackets when we have two or more of the same polyatomic ions in the formulae.
(vii) Tin(IV) Oxide
| Symbol | Sn | O |
| Charge | +4 | -2 |
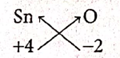
Formula : Sn2O4 or SnO2
Note : All subscripts must be reduced to lowest term (except for molecule or covalent compound),
Molecular Mass :
The molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule of the substance. It is therefore, the relative mass of a molecule expressed in atomic mass units (u).
Examples :
Calculate the molecular mass of,
(i) Water (H2O) :
The relative molecular mass of water (H2O) is 18 u, which can be calculated as,
atomic mass of hydrogen = 1 u
atomic mass of oxygen = 16 u
H2O contains two hydrogen atoms and one oxygen atom.
Therefore, molecular mass of water is = 2 × 1 + 1 × 16 = 18 u
(ii) Ammonia (NH3)
Atomic mass of hydrogen = 1 u
Atomic mass of nitrogen = 14 u
So, the molecular mass of ammonia, which contains three
atoms of hydrogen and one atom of nitrogen is = 1 × 14 × 3 × 1 = 17 u
Similarly,
(ii) Molecular mass of hydrochloric acid (HCl) = 1 x 1 + 1 x 35.5 = 36.5u
(iii) Molecular mass of phosphorus molecule (P4) = 4 × 31 = 124 u
(iv) Molecular mass of hydrogen molecule (H2) = 2 x 1 = 2 u
(v) Molecular mass of oxygen molecule (O2) = 2 x 16 = 32 u
(vi) Molecular mass of sulphur dioxide(SO2) = 1 x 32 + 2 × 16 = 64 u
Formula unit mass : It is the sum of the atomic masses of all atoms present in a formula unit of a compound.
Formula unit mass is calculated in the same manner as we calculate the molecular mass.
The only difference is that, here the word formula unit is used for the substance whose constituent particles are ions.
e.g. Formula unit mass for sodium chloride (NaCl) = 1 x 23 + 1 × 35.5 = 58.5 u
Click on below links to get PDF from store
PDF : NCERT-Class 9-Science-Chapter-3-Atoms and Molecules-Notes
PDF : NCERT-Class 9-Science-Chapter-3-Atoms and Molecules-Solution
Main Page : NCERT-Class-9-Science – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-2-Is Matter Around Us Pure? – Online Notes
Next Chapter : Chapter-4- Structure of the Atom – Online Notes
We reply to valid query.