Matter in our Surroundings
NCERT-Class-9-Science-Chapter-1
Notes
|
Topics to be learn :
|
Introduction :
- Matter, the material that occupies space and has both mass and volume, is present in our surroundings in various forms such as air, food, clothing, stones, clouds, stars, plants, animals, and even a small drop of water or sand.
- Matter can be seen, tasted, smelled, or felt, and is the basis of everything in the universe.
- Early Indian philosophers classified matter into five basic elements, called Panch-Tatva, including air, water, earth, sky, and fire.
- Today, matter is classified into groups based on physical properties and chemical nature, such as solid, liquid, gas, elements, compounds, and mixtures, based on particle arrangement or chemical nature.
Physical Nature of Matter :
Matter is made up of particles : How small are these particles of matter?
The study of matter's physical composition reveals that it consists of distinct particles with varying shapes, sizes, and natures, with these particles being very small or tiny, (beyond our imagination).
Characteristics of Particles of Matter :
Some important characteristics of particles of matter are as follows
- Particles of matter have space between them. These spaces in between the particles are called inter particle spaces.
- Particles of matter are in a state of continuous movement. This suggests that they possess some energy, called the kinetic energy. As the temperature rises, the kinetic energy of the particles increases and hence, particles move faster.
- Particles of matter have a tendency to intermix on their own with each other. They do so by getting into the spaces between the particles. This intermixing of particles of two different types of matter on their own is called diffusion.
- A force of attraction exists between the particles. This force keeps the particles together. The strength of this force of attraction varies from one kind of matter to another.
States of Matter :
Matter around us exists in three different states which are solid, liquid and gas. These states of matter arise due to the variation in the characteristics of the particles of matter.
The solid state :
Solid is defined as that form of matter in which constituent particles are held very close to each other in an orderly fashion. As a result of which, less or negligible movement occurs between their particles.
Magnified schematic picture of solid state is shown below :
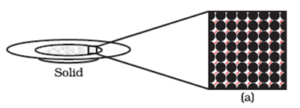
Because of such arrangement of particles, solid states of matter exhibits the following properties.
- Solids have a definite shape, distinct boundaries, and fixed volumes, making them negligible compressible. Sugar and salt are solids because their crystal shape remains fixed. Sponge is compressible but solid due to its minute holes, where air is trapped. When pressed, the air is expelled, allowing for compression.
- They have a tendency to maintain their shape when subjected to outside force. e.g. A rubber band changes its shape under force and regains the same shape when the force is removed. If excessive force is applied, it breaks.
- Solids may break under force, but it is difficult to change their shape, so they are considered as rigid.
- Generally, solids have higher densities as compared to their liquid or gaseous forms as they have very small interparticle spaces.
Note : Mass per unit volume of a substance is called density.
The liquid state :
Liquid is a form of matter where particles are arranged close together, allowing them to move but in a restricted way.
Magnified schematic picture of liquid state :
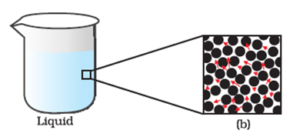
Because of such arrangement of particles, liquid state exhibits the following properties.
- Liquids do not have a definite shape, i.e. they take up the shape of the container in which they are kept.
- Liquids can flow and change shape, so they are not rigid, but can be called fluid.
Note : In science, the common name of gases and liquids is fluid.
- The diffusion of solids, liquids, and gases into liquids, particularly oxygen and carbon dioxide, is crucial for the survival of aquatic animals and plants, as they can breathe under water due to the presence of dissolved oxygen.
- Liquids are almost incompressible.
- The attraction force between the particles of liquid is greater than that of gases, but less than that of solids.
- The rate of diffusion of liquids is higher than that of solids. This is due to the fact that in the liquid state, particles move freely and have greater spaces between them as compared to particles in the solid state.
- Density of a liquid is generally less than that of its solid form. Some exceptions are also there, e.g. solid ice is lighter than water as it floats on water.
The gaseous state :
Gases are matter with dispersed particles compared to solid or liquid states, allowing for easy and fast movement of constituent particles due to their distance from each other.
Magnified schematic picture of the gaseous state is shown below :
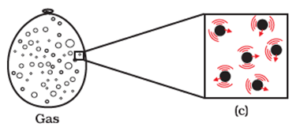
Because of such arrangement of particles, gaseous state exhibits the following characteristics.
- Gases have a tendency to flow as liquids do. Therefore, they are also considered as fluids.
- Gases show the property of diffusing very fast into other gases due to high speed of particles and large spaces between them.
- Due to the high diffusion tendency of gases, the smell of hot cooked food reaches us in seconds. The particles of the aroma of food mix with the particles of air spread, reach us and even farther away.
- Gases are highly compressible. The Liquefied Petroleum Gas (LPG) cylinder used in our homes for cooking and the oxygen supplied to hospitals in cylinders is compressed gas.
- Compressed Natural Gas (CNG) is used as a fuel these days in vehicles.
- In gaseous state, the particles move randomly at high speed. Due to this random movement, gases exert pressure on the walls of the container in which they are kept.
- The density of gases is minimum as compare to other states. A gas is much lighter than the same volume of a solid or a liquid.
Comparison of Three States of Matter :
| Property | Solid state | Liquid state | Gaseous state |
| Shape | Fixed shape | No fixed shape | No fixed shape |
| Compressibility | Can't be compressed | Can't be compressed easily | Can be compressed |
| Nature | Rigid | Fluid | Highly fluid |
| Diffusion | Negligible | Slow | Very fast |
Can Matter Change its State?
In daily life, substances exist in three states: solid, liquid, and gas. Water, for example, is the most common, forming ice, water, and water vapor. These states are interconvertible, allowing for the understanding of matter's properties.
Following two factors (or any one of these) make it possible to convert one state of matter into another: (i) Change in temperature. (ii) Change in pressure.
Terms Involved in Change of State :
The following terms are involved in change of state.
(1) Fusion (or Melting) and Melting Point :
The process of conversion of a matter from its solid state to liquid state (usually by heating) is called fusion/melting.
- The minimum temperature at which a solid to become a liquid at the atmospheric pressure is called the melting point of that solid. e.g. Melting point of ice is 0 0C or 273.15 K.
- The melting point of a solid indicates the strength of the force of attraction between its particles. Higher the melting point of a solid stronger are the forces of attraction between the constituents particles.
(2) Boiling and Boiling Point :
The process of conversion of a matter from its liquid state to vapours (gaseous state) at specific conditions of temperature and pressure is called boiling. The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
- Boiling is a bulk phenomenon because in this, the particles of the bulk of liquid gain energy and then get converted into gaseous or vapour state.
- The boiling point of water is 373 K or 100 0
(3) Vaporisation :
The process of conversion of a matter from its liquid state to gaseous state at specific conditions of temperature and pressure is called vaporisation.
(4) Sublimation :
The process of change of solid state directly into gaseous state without passing through the liquid state is known as sublimation.
- The direct change of gas to solid without changing into liquid is called deposition.
- g. Naphthalene, camphor, iodine, ammonium chloride, etc., are the solids that undergo sublimation.
(5) Freezing and Freezing Point :
The process of conversion of matter from its liquid state to solid state at specific conditions of temperature and pressure is called freezing. It is a reverse process of
fusion/melting.
- The definite temperature at which a liquid changes into solid state by giving out heat energy at 1 atm is called the freezing point.
(6) Condensation :
The process of conversion of matter from its gaseous state to liquid state at specific conditions of temperature and pressure is called condensation. It is a reverse process of vaporisation.
Effect of change of temperature :
- When a solid is heated, its particles' kinetic energy increases, leading to greater vibration and breaking free from attraction. This energy overcomes the forces of attraction, causing the particles to move more freely.
- The particles leave their fixed positions and start moving more freely. At a certain stage (i.e. at melting point), solid melts and is converted into a liquid state.
- At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other. At this temperature (i.e. boiling point), the liquid starts changing into gas.
- Conversely, by cooling, a gas can be converted into a liquid state and a liquid into a solid state.
Effect of change of temperature on the physical state may be summarised as

Difference between Gas and Vapour :
| Gas | Vapour |
| A substance is said to be a gas if its boiling point is below room temperature, e.g. O2, N2, CO2, etc. | If the normal physical state of a substance is either a solid or a liquid, but gets converted into the gaseous state either on its own or by absorbing energy, that gaseous state is called the vapour state, e.g. vapours of water in air. |
Scales of Measuring Temperature :
Kelvin (K) is the SI unit of temperature.
- Temperature on Kelvin scale = Temperature on Celsius scale + 273
- Temperature on Celsius scale = Temperature on Kelvin scale -273 0C = 273.15 K ~ 273 K
Latent Heat :
The heat energy which has to be supplied to change the state of substance is called its latent heat.
- In actual, the word 'latent' means 'hidden'.
- Latent heat does not raise (or increase) the temperature.
Latent heat is of the following two types :
(i) Latent Heat of Fusion (Solid to Liquid Change) : The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure and at its melting point is known as the latent heat of fusion.
- Particles in water at 0 ℃ (273.16 K) have more energy as compared to particles in ice at the same temperature.
(ii) Latent Heat of Vaporisation (Liquid to Gas Change) :
The amount of heat energy that is required to convert 1 kg of a liquid into gas (at its boiling point) without any rise in temperature is known as the latent heat of vaporisation.
- Particles in steam, i.e. water vapours at 373 K (100 ℃) have more energy than water at the same temperature.
Effect of change of pressure :
The physical state of a substance can be altered by adjusting pressure. An increase in pressure brings particles closer, increasing the force of attraction between them. For instance, when high pressure is applied to a gas, it is converted to a liquid, liquefied.
By applying pressure, particles of matter can be brought close together
- g. Gaseous carbon dioxide, also known as dry ice, can be stored in solid form under high pressure. When pressure decreases to 1 atmosphere, solid CO2 converts directly into gaseous state,
- Thus, we can conclude that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.

Evaporation :
- Evaporation is the conversion of a liquid into its vapour state at any temperature below its boiling point.
- Surface particles have higher kinetic energies than bulk particles, allowing them to break away from the attraction of other particles and convert into vapor. Water, when left uncovered, slowly changes into vapor.
Factors affecting evaporation :
The rate of evaporation of a liquid depends upon the following factors
- Surface area : Evaporation is a surface phenomenon, if the surface area is increased, the rate of evaporation incrcascs. e.g. While putting clothes for drying up we spread them out.
- Temperature : The rate of evaporation of a liquid increases with a rise in temperature. With the increase of temperature, more number of particles get enough kinetic energy to go into vapour state. That is why, evaporation is faster in a hot summer day than in winter or on a cloudy day.
- Humidity : It is the amount of water vapour present in air. The air around us cannot hold more than a definite amount of water vapour at a given temperature. If the humidity is high, the rate of evaporation decreases. That is why, clothes dry up faster on a dry day than on a wet (rainy) day.
- Wind speed : We know that clothes dry faster on a windy day. This is because with increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surroundings.
How does evaporation cause cooling?
In an open vessel, liquid evaporation occurs as particles absorb energy from the surrounding to regain lost energy, causing the surroundings to become cold.
Some daily life examples of cooling effect of evaporation are given below :
(i) When ice-cold water is kept in a glass tumbler for some time, water droplets are observed on its outer surface.
- Explanation : This occurs when air vapors condense with the tumbler's surface, forming small water droplets. The formation of water droplets on the tumbler's outside surface indicates the presence of water vapor in the air.
(ii) Cotton clothes are preffered to wear during summer season.
- Explanation : Cotton is a good absorber of water, so it helps to absorb sweat from our body. As it is obvious, the person sweats more during summer due to auto temperature control mechanism. Hence, wearing of cotton clothes helps in the easy evaporation of sweat. When this sweat evaporates, it takes the latent heat of vaporisation from our body, which in turn, cools the body. Thus, a person feels comfortable.
(iii) People sprinkle water on the roof or open ground on a hot sunny day.
- Explanation : When water is sprinkled on a hot surface, it gets evaporated very quickly. As evaporated water leaves the surface cool due to the large latent heat of vaporisation of water, this technique is quite effective in summers for cooling the surface.
(iv) Liquids like acetone (nail-polish remover) or alcohol placed on your palm give you feeling of cooling.
- Explanation : Acetone and alcohol are volatile liquids. When kept on palm, their particles gain energy from the palm or surroundings and evaporate, causing the palm to feel cool.
[Volatile liquids : The liquids which evaporate fast at room temperature are called volatile liquids.]
Click on below links to get PDF from store
PDF : Class 9th-Science-Chapter-1-Matter in our Surroundings-Notes
PDF : Class 9th-Science-Chapter-1-Matter in our Surroundings-Solution
Main Page : NCERT-Class-9-Science – All chapters notes, solutions, videos, test, pdf.
Next Chapter : Chapter-2- Is Matter Around Us Pure? – Online Notes
We reply to valid query.