Particulate Nature of Matter
NCERT-Class-8-Science (Curiosity)-Chapter-7
Solutions
NCERT Probe & Ponder Questions :
Question 1. Why is it possible to pile up stones or sand, but not a liquid like water?
- Solids like stones or sand can be piled up because their particles are tightly packed, strongly attracted, and stay in fixed positions. This gives solids a definite shape and allows them to form a stable heap.
- Liquids like water cannot be piled up because their particles are loosely packed, have weaker attraction, and can move past each other. Liquids have no fixed shape and always flow and spread out, so they cannot form a pile.
Question 2. Why does water take the shape of folded hands but lose that shape when released?
Water takes the shape of your folded hands because a liquid always fits the shape of whatever container it is in. But the moment you open your hands, the water loses that shape.
This happens because:
- Water particles can move and slide past each other.
- The forces of attraction between liquid particles are not strong enough to hold a fixed shape.
Since liquids flow and cannot support their own shape, the water spreads out—just like when it spills on a table.
Question 3. We cannot see air, so how does it add weight to an inflated balloon?
How unseen air adds weight
- Air is matter, and all matter has mass.
- Air is made of tiny, invisible particles.
Why an inflated balloon becomes heavier.
- When you blow air into a balloon, you are filling it with many tiny air particles. Each particle has a little mass. When many particles collect inside the balloon, they increase its total mass.
That is why an inflated balloon weighs more than an empty balloon.
Question 4. Is the air we breathe today the same that existed thousands of years ago?
- Yes, the air we breathe today is mostly the same air that existed thousands of years ago. The particles of air (like oxygen, nitrogen, and carbon dioxide) keep moving around the Earth in a natural cycle. They get used, released, and reused again and again.
- Plants, animals, oceans, and weather all help recycle the same air. So, the oxygen you breathe in today may have once been breathed out by ancient humans, dinosaurs, or even plants millions of years ago!
Intext Questions :
Question 1. Is every speck of this fine chalk powder still composed of the same substance, or has it changed into something else on breaking or grinding? (Page 99)
Yes, grinding or breaking chalk does not form a new substance because it is a physical change. Only the size of the chalk pieces becomes smaller. The tiny particles that make up the chalk remain the same. So even though the chalk turns into powder, its chemical identity does not change.
Question 2. Are the units of chalk obtained in this manner considered the smallest units of chalk? (Page 100)
No, the obtained units of chalk in the process of grinding is not the smallest
unit. Every unit of chalk is even consist of constituent particles which is the basic units of chalk.
Question 3. Do gases also have a fixed volume? (Page 105)
No, gases don't have fixed shape or volume. The volume of gas changes with the amount of closeness of particles or interparticle attraction between particles.
Question 4. Sugar and sand are both solids. Why does sugar dissolve in water but sand does not? (Page 108)
- Sugar particles are solid but it dissolves in water and occupy some space between the water molecules. Because water can breaks down sugar particles which reduces the total volume of mixture.
- Whereas sand particles have rigid crystal structure which cannot be broken down by water molecules and hence settles down in water and increases the total volume.
Activity 7.9: Let us find out (Page 110)
- Light an incense stick in one corner of the room.
- Wait for a few minutes and observe.
- Do you notice the fragrance from a distance?
Observations:
- We can smell the fragrance of incense stick from one corner.
- Slowly the fragrance of this incense stick can be smell all around the room.
Conclusions:
- The smell of incense stick can be observed throughout the room because the particles of air are moving randomly.
- As the particles interparticles forces are weak, so they are able to move freely around room.
Exercise Questions : Keep The Curiosity Alive :
Question 1. Choose the correct option.
The primary difference between solids and liquids is that the constituent particles are:
(i) closely packed in solids, while they are stationary in liquids.
(ii) far apart in solids and have fixed position in liquids.
(iii) always moving in solids and have fixed position in liquids.
(iv) closely packed in solids and move past each other in liquids.
(iv) closely packed in solids and move past each other in liquids.
Question 2. Which of the following statements are true? Correct the false statements.
(i) Melting ice into water is an example of the transformation of a solid into a liquid.
True: Melting ice into water is an example of the transformation of a solid into a liquid.
(ii) Melting process involves a decrease in interparticle attractions during the transformation.
True: Melting process involves a decrease in interparticle attraction during the transformation.
(iii) Solids have a fixed shape and a fixed volume.
True: Solids have a fixed shape anda fixed volume.
(iv) The interparticle interactions in solids are very strong, and the interparticle spaces are very small.
True: The interparticle interactions in solids are very strong and the interparticle spaces are very small.
(v) When we heat camphor in one corner of a room, the fragrance reaches all corners of the room.
True: When we heat camphor in one corner of a room, the fragrance reaches all corners of the room.
(vi) On heating, we are adding energy to the camphor, and the energy is released as a smell.
False: (On heating, energy is added to camphor, causing it to undergo sublimation. The camphor directly convert into gas and the vapour carries its characteristic smell.)
Question 3. Choose the correct answer with justification. If we could remove all the constituent particles from a chair, what would happen?
(i) Nothing will change.
(ii) The chair will weigh less due to lost particles.
(iii) Nothing of the chair will remain.
Correct option : (iii) Nothing of the chair will remain.
Justification:
A chair is made up of constituent particles (atoms and molecules). If you remove all the particles from the chair there is nothing left to form the structure, shape, weight or existence of the chair.
Question 4. Why do gases mix easily, while solids do not?
Gases and solids mix differently because their particles behave very differently.
(i) Why gases mix easily:
Gas particles are very far apart, move freely in all directions, and have almost no attraction between them.
- There is lots of empty space for other gas particles to enter.
- Gas particles do not stick to each other.
- They move quickly and spread out on their own.
Because of this, one gas can easily and completely mix with another gas.
(ii) Why solids do not mix easily:
Solid particles are tightly packed and held together by strong attractive forces.
- Their particles stay fixed in place.
- They can only vibrate, not move around.
Since solid particles cannot move freely or change positions, the particles of two solids cannot easily mix with each other.
Question 5. When spilled on the table, milk in a glass tumbler, flows and spreads out, but the glass tumbler stays in the same shape. Justify this statement.
Difference between liquid (milk) and solid (glass tumbler):
- Shape and Volume: Milk has a fixed volume but no fixed shape. It takes the shape of any container. A glass tumbler has both fixed shape and fixed volume.
- Particle Attraction: In liquids like milk, particles are not tightly held, so they can move and flow. In solids like glass, particles are strongly attracted and tightly packed.
- Particle Movement: Liquid particles move freely within a limited space. Solid particles cannot move freely; they only vibrate in place.
Therefore,
- Milk spreads when spilled because its particles can move.
- A glass tumbler keeps its shape because its particles are tightly packed and fixed.
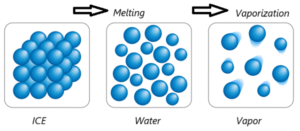
Question 6. Represent diagrammatically the changes in the arrangement of particles as ice melts and transforms into water vapour.
As ice melts into water and then vaporizes into steam, the arrangement of water particles changes significantly.
Fig. shows how particles rearrange themselves as ice melts into water and then evaporates into vapour.
- Ice (Solid): Particles are closely packed in a fixed, orderly arrangement. They only vibrate in place.
- Water (Liquid): Particles are still close but loosely arranged. They can slide past each other, giving water fluidity.
- Water Vapour (Gas): Particles are far apart and move randomly at high speed, filling the available space.
Question 7. Draw a picture representing particles present in the following:
(i) Aluminium foil
(ii) Glycerin
(iii) Methane gas
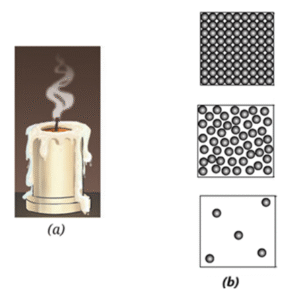
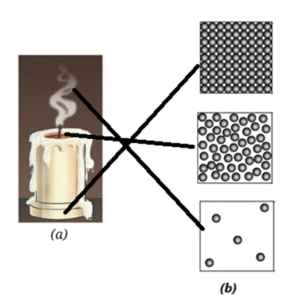
Question 8. Observe Fig. (a) which shows the image of a candle that was just extinguished after burning for some time. Identify the different states of wax in the figure and match them with Fig. (b) showing the arrangement of particles.
Question 9. Why does the water in the ocean taste salty, even though the salt is not visible? Explain.
Ocean water tastes salty because salt is dissolved in it, even though we cannot see it.
- Ocean water has dissolved salts like sodium chloride.
- When salt dissolves, it breaks into tiny particles that spread throughout the water.
- These particles fill the spaces between water molecules, so they cannot be seen.
- The salt does not settle or form clumps, making the water look clear.
- Even though it is invisible, we can taste the salt in the water.
Question 10. Grains of rice and rice flour take the shape of the container when placed in different jars. Are they solids or liquids? Explain.
Grains of rice and rice flour are considered solids even though they seem to take the shape of the container.
They are solids because,
- Each grain or speck has its own fixed shape and fixed volume.
- The particles inside each grain are tightly packed with strong forces of attraction.
They appear to flow because,
- When many tiny solid particles are together, they can slide past each other.
- Because of this, the whole mass can settle and spread to fill the container’s shape.
- This is similar to sand or small stones — each piece is solid, but the group behaves like it can flow.
Key Features of Kitabcd Exam Master :
|
Click on below links to get PDF from store
PDF : Class 8 -Curiosity-Chapter-7-Particulate Nature of Matter– Notes
PDF : Class 8 -Curiosity-Chapter-7-Particulate Nature of Matter– Exam Master
PDF Set :
Class -8-Science (Curiosity) -All 13 Chapters Notes Set (13-PDF)-Rs.58
Class -8-Science (Curiosity) -All 13 Chapters Exam Master Set (13-PDF)-Rs.74
Class -8-Science (Curiosity) -All 13 Chapters Notes + Exam Master Set (26-PDF)-Rs.116
Main Page : NCERT-Class-8-Science (Curiosity) – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6- Pressure, Winds, Storms, and Cyclones – Online Solutions
Next Chapter : Chapter-8- Nature of Matter: Elements, Compounds, and Mixtures – Online Solutions

We reply to valid query.