The Amazing World of Solutes, Solvents, and Solution
NCERT-Class-8-Science (Curiosity)-Chapter-9
Notes
Mixtures and Solutions :
The physical world involves numerous instances of substances mixing. These mixtures can be categorized based on the uniformity of their component distribution.
Uniform vs. Non-Uniform Mixtures :
Uniform Mixtures (Solutions): In these mixtures, the components are evenly distributed throughout, resulting in a consistent composition. Every part of the mixture has the same properties.
Examples :
- Oral Rehydration Solution (ORS), where salt and sugar dissolve completely in water, making every sip taste the same.
- Air, a mixture of gases, is another example of a uniform mixture.
Non-Uniform Mixtures: In these mixtures, the components are not evenly distributed and can often be seen separately, either with the naked eye or a magnifying device.
Examples :
- Mixing chalk powder, sand, or sawdust with water, where the solid particles settle or float rather than dissolving.
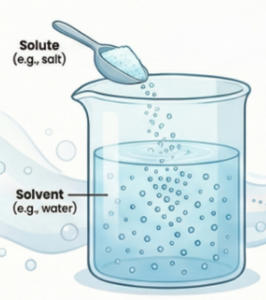
What Are Solute, Solvent, and Solution? :
Solution: A uniform mixture (homogeneous mixture) of two or more substances. The particles in a solution are so small they cannot be distinguished from the solvent.
- Example: Air, which is a uniform mixture of gases.
Solute: The substance present in a smaller amount in a solution, which gets dissolved by the solvent.
- Examples: Salt in saltwater, sugar in sugar water, oxygen in air.
Solvent: The component present in a larger amount in a solution that does the dissolving.
- Examples: Water in saltwater, nitrogen in air. (In air mixture, nitrogen being present in large quantity is solvent and the other components present like oxygen, argon, carbon dioxide are solutes.)
Aqueous Solution: A solution in which water acts as the dissolving medium. Water is often called the universal solvent because it can dissolve more substances than any other liquid.
Types of Solutions :
Solutions can be formed from different states of matter. Three primary types of mixtures that can form solutions if they are uniform:
- Solid in liquid
- Liquid in liquid
- Gas in gas
How Much Solute Can a Fixed Amount of Solvent Dissolve?
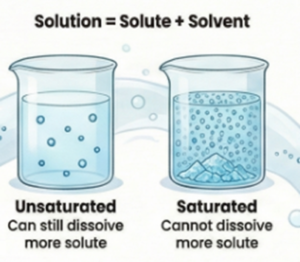
Saturation Levels :
- Unsaturated Solution: A solution that can dissolve more solute at a given temperature.
- Saturated Solution: A solution that contains the maximum amount of solute that can be dissolved at a given temperature. No more solute can be dissolved into the saturated solution at that temperature; any additional solute will settle at the bottom.
Relationship with Temperature:
- A saturated solution becomes unsaturated when its temperature is raised, as the solvent can then hold more solute.
- Conversely, an unsaturated solution can become saturated if its temperature is lowered.
Concentration : The amount of solute present in a fixed quantity of solution.
- Dilute Solution: Contains a small amount of dissolved substance.
- Concentrated Solution: Contains a higher amount of dissolved substances.
Example: A solution of one teaspoonful of salt in 100 mL of water is dilute compared to a solution of two teaspoonfuls of salt in 100 mL of water.
Solubility :
Solubility is a measure of the maximum amount of a solute that can dissolve in a fixed quantity of a solvent at a specific temperature. It determines how much solute is needed to form a saturated solution.
Factors Affecting Solubility :
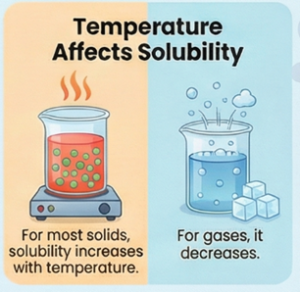
(i) Temperature:
- Solids in Liquids: The solubility of most solids increases with a rise in temperature. An experiment demonstrates that water at 70°C dissolves more baking soda than water at 50°C.
- Gases in Liquids: The solubility of gases decreases as temperature increases. Oxygen, for instance, is more soluble in cold water than in hot water.
(ii) Pressure:
- The effect of pressure on the solubility of solids and liquids is described as negligible.
- Pressure does affect the density of gases.
Solubility of Gases :
- Many gases, including oxygen, dissolve in water.
- The solubility of gases generally decreases as the temperature of the liquid increases.
- This is crucial for aquatic life, as cold water can hold more dissolved oxygen than warm water, ensuring sufficient oxygen for fish and other organisms to survive.
Why Do Objects Float or Sink in Water?
- The tendency of an object to float or sink is primarily explained by the property of density.
- Whether an object floats or sinks in a liquid is related to its density compared to the liquid's density.
- For example, oil floats on water because it is less dense than water.
What Is Density? :
Density is defined as the mass present in a unit volume of a substance. It is an intrinsic property, independent of the object's shape or size.
Density = Mass / Volume
The SI unit for density is kilogram per cubic metre (kg/m³). Other common units include gram per millilitre (g/mL) or gram per cubic centimetre (g/cm³).
Example : If the mass of an aluminium block is 27 g and its volume is 10 cm3, find it’s density.
Density = \(\frac{Mass}{Volume}=\frac{27}{10}\) = 2.7 g/cm³
Relative Density : It is a unitless number that compares the density of a substance to the density of water at a specific temperature.
Relative Density = Density of Substance / Density of Water
For example, an aluminum block with a density of 2.7 g/cm³ has a relative density of 2.7, indicating it is 2.7 times denser than water.
Conversion factor for density :
1 kg/m3 = 1000 g/m3 = 1000 g/1000 L = 1 g/L = 1 g/1000 mL = 1 g/1000 cm3
The mass of 1 mL of water is close to 1 g at room temperature.
Factors Affecting Density :
(i) Temperature: Generally, the density of a substance decreases with heating and increases with cooling.
- As temperature rises, particles move farther apart, increasing the volume while the mass remains constant, thus lowering the density.
- This principle is utilized in hot air balloons, which rise because the heated air inside is less dense than the cooler surrounding air.
(ii) Pressure: The effect of pressure on density varies by the state of matter.
- Gases: Increasing pressure pushes gas particles closer, decreasing volume and increasing density.
- Liquids & Solids: These are nearly incompressible, so changes in pressure have a negligible effect on their density.
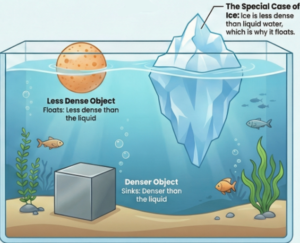
Special Case: The Density of Water and Ice :
- Water exhibits an anomalous property: it is densest at 4°C. As water cools further and freezes into ice at 0°C, its particles arrange into a crystalline structure that occupies more space. This expansion means ice has a lower density than liquid water, causing it to float.
- This phenomenon is vital for aquatic ecosystems, as the floating ice layer insulates the water below, allowing life to survive in cold climates.
Measurement of Physical Quantities :
(i) Mass and Weight :
- Mass: The quantity of matter present in an object. It is measured in grams (g) or kilograms (kg) using a digital weighing balance.
- Weight: The force with which the Earth attracts an object. It is measured in newtons (N).
(ii) Volume :
The amount of space occupied by an object.
(a) Measuring Liquid Volume:
Apparatus: A measuring cylinder, a narrow, tall, transparent container. The tall and narrow design allows for precise indication of small changes in volume.
Procedure:
- Place the cylinder on a flat surface.
- Pour the liquid in slowly.
- Read the mark that aligns with the meniscus, ensuring eye level is level with the liquid.
- For colorless liquids, read the bottom of the curved meniscus.
- For colored liquids, read the upper meniscus.
(b) Measuring Solid Volume:
- Regular Solids: Calculated using the formula: Volume = Length × Width × Height.
- Irregular Solids: Measured by the water displacement method. The object is submerged in a measuring cylinder containing water, and the volume is the difference between the final water level (V₂) and the initial water level (V₁).
Historical Applications in Medicine :
- Traditional Indian systems of medicine like Ayurveda and Siddha have used water for a long time to prepare medicines.
- They also use alcohol mixed with water, oils, ghee, and milk to take out useful substances from herbs and get better healing effects.
Scientific Contribution: Asima Chatterjee
- Asima Chatterjee was a famous Indian scientist. She used solvents and solutions to separate important chemicals from medicinal plants.
- Her research helped in making medicines for epilepsy and malaria.
- She was the first woman to receive the Shanti Swarup Bhatnagar Award in chemical science and also received the Padma Bhushan.
Traditional Practice: Salt Making in Ningel, Manipur
- In Ningel village of Manipur, people still make salt using a traditional method.
- Salty water is taken from wells, some lined with very old tree trunks. This water is boiled until it dries, leaving behind salt crystals.
- The salt is shaped into round salt cakes, wrapped in cloth, and is believed to have medicinal value.
- This practice shows the close link between food, history, and culture.
Key Features of Kitabcd Exam Master :
|
Click on below links to get PDF from store
PDF : Class 8 -Curiosity-Chapter-9-The Amazing World of Solutes, Solvents, and Solution– Notes
PDF : Class 8 -Curiosity-Chapter-9-The Amazing World of Solutes, Solvents, and Solution– Exam Master
PDF Set :
Class -8-Science (Curiosity) -All 13 Chapters Notes Set (13-PDF)-Rs.58
Class -8-Science (Curiosity) -All 13 Chapters Exam Master Set (13-PDF)-Rs.74
Class -8-Science (Curiosity) -All 13 Chapters Notes + Exam Master Set (26-PDF)-Rs.116
Main Page : NCERT-Class-8-Science (Curiosity) – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-8-Nature of Matter: Elements, Compounds, and Mixtures– Online Notes
Next Chapter : Chapter-10- Light: Mirrors and Lenses – Online Notes


We reply to valid query.