Particulate Nature of Matter
NCERT-Class-8-Science (Curiosity)-Chapter-7
Notes
Particulate nature of matter:
- Matter is not one continuous mass. It is made up of very tiny particles. This idea is called the particulate nature of matter.
- Because of these small particles, different substances show different properties and behave in different ways.
What Is Matter Composed of? :
Matter: Defined as anything that has mass and takes up space.
- Everything in the surrounding environment, including air, water, books, and rocks, is made of matter.
Constituent Particle: The smallest basic unit that forms a larger piece of a substance.
- These particles, which include atoms, molecules, ions, and subatomic particles, are in constant motion and have spaces between them.
- They are so small they cannot be seen with the naked eye or even an ordinary microscope.
Experimental evidence, such as grinding a piece of chalk into a fine powder or dissolving sugar in water, demonstrates that substances can be broken down into their constituent particles without changing their chemical nature.
Classification of Particle :
The constituent particles that make up matter can be classified into several types, primarily atoms, molecules, and ions.
| Particle Type | Definition | Key Characteristics | Examples |
| Atom | The smallest particle of an element that can take part in a chemical reaction. | It is the basic unit of matter. Many atoms cannot exist independently. | O, H, Cl |
| Molecule | The smallest particle of a substance which retains the characteristics of the substance. | Formed when two or more atoms (of the same or different elements) are chemically bonded. | O₂, H₂, Cl₂, H₂O |
| Ion | An atom or a group of atoms which has a resultant charge due to the loss or gain of electrons. | Carries a positive or negative electrical charge. | O²⁻, H¹⁺, Cl¹⁻ |
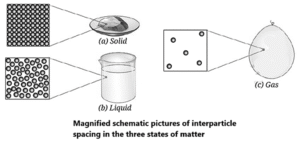
What Decides Different States of Matter? :
The state of matter is determined by the arrangement, energy, and forces between its constituent particles. The three primary states are solid, liquid, and gas.
| Property | Solid State | Liquid State | Gas State |
| Shape & Volume | Definite shape and definite volume. | Indefinite shape (takes shape of container) but definite volume. | Indefinite shape and indefinite volume (fills container completely). |
| Particle Packing | Tightly and closely packed in a regular, fixed structure. | Loosely packed compared to solids, with no fixed positions. | Particles are very far apart and randomly distributed. |
| Interparticle Spacing | Minimum; tiny gaps exist between particles. | More spacing than in solids, but particles remain relatively close. | Maximum spacing; large empty spaces between particles. |
| Interparticle Force | Maximum; very strong forces hold particles in fixed positions. | Weaker than in solids, but strong enough to keep particles together. | Minimum; attractive forces are negligible. |
| Particle Movement | Particles vibrate or oscillate about their fixed positions. | Particles can slide past one another and move within a limited space. | Particles move freely, rapidly, and randomly in all available space. |
| Kinetic Energy | Lowest kinetic energy. | Higher kinetic energy than solids. | Highest kinetic energy. |
| Compressibility | Practically incompressible. | Practically incompressible. | Highly compressible. |
| Examples | Ice, rock, wood, sugar, iron | Water, milk, oil, glycerin, blood | Air, steam, oxygen, methane gas |
Both liquids and gases are classified as fluids because their particles are able to move and flow, preventing them from retaining a fixed shape.
How Does the Interparticle Spacing Differ in the Three States of Matter? :
The different properties of solids, liquids, and gases depend on how far their particles are from each other, how strongly they attract each other, and how much energy they have. These things also change with temperature.
(i) Interparticle Spacing :
This means the distance between particles. The space between particles is empty, not filled with air. The amount of space decides how dense a substance is and whether it can be compressed.
- In gases, particles are very far apart, so gases can be compressed easily (like when you push the plunger of a syringe filled with air).
- In liquids and solids, particles are close together, so they cannot be compressed easily.
(ii) Interparticle Forces of Attraction :
These are the forces that pull particles toward each other. When particles are closer, these forces are stronger.
- In solids, the forces are very strong, so particles stay fixed in place.
- In liquids, the forces are weaker, so particles can move around.
- In gases, the forces are almost zero, so particles move freely and spread far apart.
(iii) Particle Motion and Kinetic Energy :
Particles of matter are always moving randomly. This movement is their kinetic energy, and it depends on temperature.
- Temperature tells us how much heat energy a substance has.
- When heat energy increases, the particles gain more kinetic energy and move faster.
- For example, potassium permanganate spreads faster in hot water because the hot water particles move faster and help mix it quickly.
How Particles Move in Different States of Matter?
Matter can change from one state to another, a process known as a phase change or change of state. These changes are physical, not chemical, and are reversible in most cases.
Phase Change Processes :
- Melting: Solid to Liquid (e.g., ice to water)
- Freezing: Liquid to Solid (e.g., water to ice)
- Vaporization/Boiling: Liquid to Gas (e.g., water to steam)
- Condensation: Gas to Liquid (e.g., steam to water)
- Sublimation: Solid to Gas directly (e.g., camphor or solid iodine turning to vapor)
- Deposition: Gas to Solid directly
Evaporation is a specific type of vaporization that occurs slowly at the surface of a liquid at any temperature below its boiling point.
Melting and Boiling Points :
- Melting Point: The fixed temperature at which a solid melts to become a liquid at atmospheric pressure. The melting point reflects the strength of interparticle forces; substances with stronger forces have higher melting points.
- Boiling Point: The fixed temperature at which a liquid rapidly turns into a gas (vapor) throughout its bulk at atmospheric pressure.
| Material | Melting Point |
| Ice | 0 °C |
| Urea | 133 °C |
| Iron | 1538 °C |
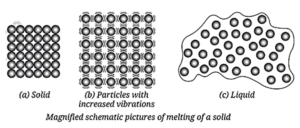
Mechanism of Phase Change Example: Ice to Steam :
The transition from solid ice to gaseous steam illustrates the role of energy:
- Solid Ice: Water molecules are locked in a regular, crystalline structure, vibrating in fixed positions.
- Melting: As heat is added, molecules gain kinetic energy and vibrate more vigorously. At the melting point (0°C), the energy overcomes the attractive forces, allowing molecules to break free from their fixed positions and slide past one another, forming liquid water.
- Liquid Water: The molecules are still close but are now mobile.
- Boiling: As heating continues, the molecules gain even more kinetic energy and move faster. At the boiling point (100°C), the particles have enough energy to overcome the remaining attractive forces entirely and escape into the gaseous state.
- Water Vapor (Steam): The molecules are far apart and move randomly and freely.
Historical Perspective: Acharya Kanad's Parmanu :
- The idea that matter is made of tiny particles is ancient.
- The Indian philosopher Acharya Kanad, in his book Vaisheshika Sutras, was the first to talk about the concept of Parmanu (atom).
- He said that everything in the world is made of very small, eternal, and indivisible particles.
Evidence and Phenomena Explained by Particulate Theory :
The particulate model successfully explains numerous everyday observations.
- Dissolution: When we mix salt or sugar in water, it dissolves because its tiny particles spread out and fit into the empty spaces between water particles. That is why the sea tastes salty even though we cannot see salt crystals. It also explains why the final volume of sugar water can be less than the separate volumes of sugar and water.
- Diffusion: Diffusion means particles spreading out. The smell of incense or camphor reaches all parts of a room because the smell particles move and mix with the moving air particles.
- State Integrity: A solid glass keeps its shape because its particles are tightly held in fixed positions. Milk flows and spreads because its liquid particles can move past one another.
- Properties of Granular Solids: Things like rice flour or sand can be poured like a liquid, but they are still solids. Each tiny grain has its own fixed shape and volume. They seem to flow because the small solid grains slide over each other.
- Existence of Objects: Any object, like a chair, exists because it is made of countless particles (atoms and molecules). If all these particles were removed, nothing would remain—no shape, no weight, nothing at all.
Glossary of Key Terms :
| Term | Definition |
| Atom | The smallest unit of an element; the basic building block of all matter. |
| Boiling point | The temperature at which a liquid boils and turns into vapour at atmospheric pressure. |
| Changes of State | The different forms that matter can take on. The change of state is reversible in most cases. |
| Constituent Particle | The smallest units that form matter. Examples include atoms, molecules, ions, and subatomic particles. A constituent particle is the basic unit that makes up a larger piece of a substance. |
| Fluids | A classification for both liquids and gases, distinguished from solids by their ability to flow and not retain a fixed shape. |
| Gases | A state of matter that has neither a fixed volume nor a fixed shape. Their particles are far apart and move freely. |
| Heat Energy | Energy that increases particle motion and can change the state of matter. More heat equals more kinetic energy in particles. |
| Interparticle attraction | The attractive forces that hold the constituent particles of matter together. The strength of these forces determines the physical state of a substance. |
| Interparticle spacing | The average distance or spaces between adjacent particles in a material. |
| Ions | An atom or group of atoms that has a resultant charge due to the loss or gain of electrons. |
| Kinetic Energy | The energy due to the motion of particles. Kinetic energy increases with temperature. |
| Liquids | A state of matter that has a fixed volume but no fixed shape. Their particles are less tightly packed than solids and can slide past one another. |
| Matter | Anything that has mass and takes up space. |
| Melting point | The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure. |
| Molecules | Two or more atoms chemically bonded together. |
| Parmanu | The term used by ancient Indian philosopher Acharya Kanad for the tiny, indivisible, eternal particles that he believed make up matter (equivalent to an atom). |
| Particle Movement (Vibration) | Particles of matter are always in motion. They vibrate (in solids), slide (in liquids), or move freely (in gases). This motion increases with heat. |
| Particles | The tiny units that make up all matter. They are so small they cannot be seen with the naked eye, are always moving, and have spaces between them. |
| Solids | A state of matter that has a fixed shape and volume. Their particles are tightly packed in fixed positions. |
| States of Matter | The different forms that matter can take on, most commonly solids, liquids, and gases. |
| Temperature | A measure of the heat energy of a substance. Higher temperature corresponds to faster particle motion and is a deciding factor in the state of matter. |
Practice Questions : (Find Answer key in Exam Master)
- What is the fundamental definition of matter?
- Contrast the arrangement and packing of particles in solids versus liquids.
- Explain why gases lack both a fixed shape and a fixed volume.
- Describe what occurs at the particle level when a solid is heated to its melting point.
- What are interparticle attractions, and how does their strength vary across the three main states of matter?
- Define interparticle spacing and explain its role in determining the state of a substance.
- What happens to sugar particles when sugar is dissolved in water?
- Differentiate between the processes of melting and boiling.
- Why does the fragrance from a burning incense stick eventually fill an entire room?
- Who was Acharya Kanad, and what was his ancient philosophical contribution to the concept of matter?
Key Features of Kitabcd Exam Master :
|
Click on below links to get PDF from store
PDF : Class 8 -Curiosity-Chapter-7-Particulate Nature of Matter– Notes
PDF : Class 8 -Curiosity-Chapter-7-Particulate Nature of Matter– Exam Master
PDF Set :
Class -8-Science (Curiosity) -All 13 Chapters Notes Set (13-PDF)-Rs.58
Class -8-Science (Curiosity) -All 13 Chapters Exam Master Set (13-PDF)-Rs.74
Class -8-Science (Curiosity) -All 13 Chapters Notes + Exam Master Set (26-PDF)-Rs.116
Main Page : NCERT-Class-8-Science (Curiosity) – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6- Pressure, Winds, Storms, and Cyclones – Online Notes
Next Chapter : Chapter-8- Nature of Matter: Elements, Compounds, and Mixtures – Online Notes
We reply to valid query.