Electricity: Magnetic and Heating Effects
NCERT-Class-8-Science (Curiosity)-Chapter-4
Notes
Does an Electric Current Have a Magnetic Effect? :
Magnetic Effect of Electric Current :
When electric current flows through a wire, it creates a magnetic field around it. This is called the magnetic effect of electric current.
Discovery :
This effect was discovered by Hans Christian Oersted in 1820. He showed that electricity and magnetism are connected.
Observation :
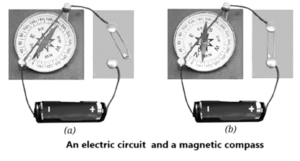
- If you place a magnetic compass near a wire carrying current, the needle moves or deflects.
- This shows that the wire produces a magnetic field.
- When the current is switched off, the needle goes back to its original position.
- So, the magnetic effect is present only when current flows.
Magnetic Field: The region around a magnet or a current-carrying wire where its magnetic effect can be felt is called a magnetic field.
Practical Applications of Magnetic Effect of Electric Current :
The magnetic effect of electric current is used in many devices we see and use every day.
Common Examples:
- Electromagnets
- Electric bells
- Electric motors
- Ceiling fans
- Loudspeakers
- Battery-operated toys
These devices work because electric current creates a magnetic field that helps them move or make sound.
Industrial Use of Electromagnets :
Electromagnets are very useful in industries, especially where magnetic materials like iron are handled.
Example:
- In factories, iron scrap is melted in hot furnaces to make iron rods and ingots.
- This job is dangerous for humans.
- So, cranes with strong electromagnets are used to lift and drop iron scrap into the furnace safely.
Electromagnets :
Electromagnets are coils of wire that act like magnets when electric current flows through them.
- To make them stronger, an iron nail or iron core is placed inside the coil.
Controllability :
Electromagnets are temporary magnets.
- When current flows, they behave like magnets.
- When current stops, the magnetic effect disappears.
- This is useful in machines like factory cranes that lift iron scrap:
- Current ON → magnet lifts objects
- Current OFF → magnet drops objects
Polarity :
Electromagnets have North and South poles, just like permanent magnets.
- You can reverse the poles by changing the direction of the current.
Increasing Strength of Electromagnets :
You can make an electromagnet stronger by:
- Using more electric current (e.g., more battery cells)
- Adding more turns of wire in the coil
- Inserting an iron core (like an iron nail) inside the coil
Lifting electromagnets :
Lifting electromagnets are powerful electromagnets used to handle heavy metal objects like iron and steel.
How They Work:
- They are attached to cranes.
- The crane operator controls them by switching the electric current ON and OFF.
- Current ON → magnet lifts metal objects
- Current OFF → magnetic field disappears, objects are released
Where They’re Used:
- Factories
- Scrap yards
- Used to move, lift, and sort heavy metal items quickly and safely
Earth's Magnetism:
The Earth acts like a big magnet. This is because:
- Inside the Earth, liquid iron moves in the outer core.
- This movement creates electric currents.
- These currents produce a magnetic field around the Earth.
Importance of Earth’s Magnetic Field :
- Helps migratory animals (like birds and turtles) to navigate.
- Acts as a protective shield against harmful particles from space (like solar wind and cosmic rays).
Does a Current Carrying Wire Get Hot? :
Heating Effect of Electric Current :
When electric current passes through a wire (called a conductor), it faces resistance. This resistance turns some of the electrical energy into heat. This is called the heating effect of electric current.
How It Works (Mechanism) :
- As current flows through a wire, it meets resistance.
- This resistance slows down the flow and produces heat.
- More resistance = more heat.
Factors That Affect Heat Generation :
- Material of Wire: Different materials resist current differently.
Example: Nichrome wire produces more heat than copper wire of the same size. - Amount of Current: More current = more heat.
- Thickness and Length of Wire:
- Thin wires have more resistance → more heat.
- Longer wires also increase resistance.
- Time Duration: The longer the current flows, the more heat is produced.
Uses of Heating Effect :
- Home Appliances: Electric heaters, irons, stoves, immersion rods, kettles, hair dryers. These have a heating element (a coil or rod) that gets hot and glows.
- Industry: Used in furnaces to melt and recycle scrap steel.
Safety Concerns :
- Too much heat can cause:
- Energy loss in wires.
- Overheating of appliances.
- Melting of plugs/sockets.
- Fire hazards.
Use proper wires and plugs to stay safe.
How Does a Battery Generate Electricity? :
Batteries, or cells, are devices that generate electric current through internal chemical reactions.
Voltaic cell :
Voltaic cells are early types of electric cells that produce electricity through chemical reactions.
Historical Background :
- Named after Alessandro Volta and Luigi Galvani.
- Their experiments showed that electricity can be made using metals and liquids—not just from living tissues.
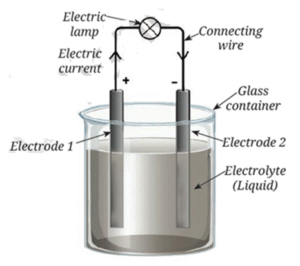
Voltaic cell - Main Parts :
- Electrodes: Two metal plates made of different materials (e.g., copper and iron).
- Electrolyte: A liquid like weak acid or salt solution.
- The metal plates are partly dipped in the electrolyte.
How It Works :
- A chemical reaction happens between the electrodes and the electrolyte.
- This reaction produces electricity.
- Current flows from the positive terminal to the negative terminal.
Lifespan :
- Over time, the chemicals get used up.
- Once finished, the cell stops working and is called “dead.”
Lemon Cell Experiment :
Purpose: To show how a voltaic cell works using everyday items like lemons, nails, and wires.
Materials Needed :
- Juicy lemons (cut or whole)
- Iron nails (act as one electrode)
- Copper wires or strips (1–2 mm thick, act as the other electrode)
- Connecting wires
- Small LED bulb
How It Works :
- Lemons contain citric acid, which acts as the electrolyte.
- Iron and copper are electrodes.
- When inserted into the lemon and connected properly, a chemical reaction occurs.
- This reaction produces a small electric current.
- If the circuit is complete, the LED glows—showing that the lemon is working like a battery.
Troubleshooting :
- If the LED doesn’t glow:
- Try reversing the terminals (swap the wires).
- Make sure all connections are tight and clean.
- Use multiple lemons in series to increase voltage.
Dry cells :
Why Dry Cells? : Voltaic cells use liquid electrolytes, which can spill and are hard to carry. Dry cells solve this problem—they are compact, safe, and easy to use.
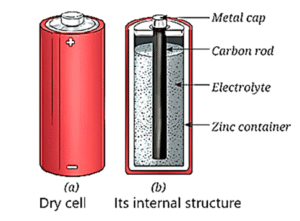
Structure of a Dry Cell :
- Zinc Container: Acts as the negative terminal and holds everything inside.
- Electrolyte Paste: A thick, moist paste (not liquid) that helps in chemical reactions.
- Carbon Rod: Placed in the center of the paste. The top of the carbon rod is covered with metal and acts as the positive terminal.
How It Works :
- A chemical reaction occurs between the zinc and the electrolyte.
- This produces electricity.
- Current flows from the positive terminal (carbon rod) to the negative terminal (zinc container).
Lifespan and Disposal :
- Once the chemicals are used up, the dry cell stops working.
- It cannot be reused and must be disposed of properly.
Rechargeable batteries :
Rechargeable batteries can be used again and again by recharging them after use. They are becoming very popular.
Uses :
- Small Devices: Smart watches, mobile phones, wireless accessories.
- Large Devices: Laptops, inverters (backup power), motor vehicles.
Lifespan :
- These batteries don’t last forever.
- After many charge–use cycles, they wear out and must be replaced.
Advanced Types :
- Lithium-ion Batteries: Used in most modern devices. Give better performance and last longer.
- Solid-State Batteries (Future Tech): Use solid electrolytes instead of liquids. Expected to be safer, charge faster, and last longer.
Why Are They Important? :
- Rechargeable batteries help reduce waste and support a cleaner environment.
- Better portable energy sources are needed for a safe and sustainable future.
Battery Disposal and Recycling:
- Even "dead" batteries contain materials like acids and metals (lead, cadmium, nickel, lithium) that can be harmful to the environment if improperly discarded.
- Recycling facilities for "e-waste" are crucial for recovering valuable materials and mitigating environmental impact.
Glossary of Key Terms :
- Magnetic Effect of Electric Current: The phenomenon where an electric current flowing through a conductor produces a magnetic field around it.
- Magnetic Field: The region around a magnet or a current-carrying wire where its magnetic effect can be felt, such as by the deflection of a compass needle.
- Electromagnet: A current-carrying coil that behaves as a magnet. Its magnetic properties are temporary and can be controlled by switching the current on and off.
- Heating Effect of Electric Current: The phenomenon where a conductor gets heated up when electric current passes through it due to its resistance to the current flow.
- Resistance: The opposition a conductor offers to the flow of electric current, converting some electrical energy into heat energy.
- Heating Element: A rod or coil of wire found in heating appliances that generates heat when electric current passes through it.
- Voltaic Cell (Galvanic Cell): One of the earliest types of electric cells, which generates electricity through a chemical reaction between two different metal plates (electrodes) and an electrolyte (a weak acid or salt solution).
- Electrodes: The metal plates in an electric cell that participate in the chemical reaction to produce electricity.
- Electrolyte: The liquid or paste-like substance in an electric cell that conducts electricity and participates in the chemical reactions.
- Dry Cell: A widely used electric cell where the electrolyte is a thick, moist paste instead of a liquid. It is a single-use cell.
- Rechargeable Battery: A type of battery that can be recharged and reused multiple times, making it more sustainable than single-use cells.
- Lithium-ion (Li-ion) Battery: The most common type of rechargeable battery today, found in many electronic devices, relying on special metals like lithium and cobalt.
- Solid-state Batteries: Future battery technology that replaces liquid or paste-like electrolytes with solid materials, aiming for greater safety, faster charging, and longer lifespan.
Key Features of Kitabcd Exam Master :
|
Click on below links to get PDF from store
PDF : Curiosity-Chapter-4-Electricity: Magnetic and Heating Effects– Notes
PDF : Curiosity-Chapter-4-Electricity: Magnetic and Heating Effects– Exam Master
PDF Set :
Class -8-Science (Curiosity) -All 13 Chapters Notes Set (13-PDF)-Rs.58
Class -8-Science (Curiosity) -All 13 Chapters Exam Master Set (13-PDF)-Rs.74
Class -8-Science (Curiosity) -All 13 Chapters Notes + Exam Master Set (26-PDF)-Rs.116
Main Page : NCERT-Class-8-Science (Curiosity) – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-3- Health: The Ultimate Treasure – Online Notes
Next Chapter : Chapter-5- Exploring Forces – Online Notes

We reply to valid query.