Exploring Substances: Acidic, Basic, and Neutral
NCERT-Class-7-Science (Curiosity)-Chapter-1
Solutions (Exercise + Intext)
Intext Questions
Question 1: Find out and write the names of the most common acids present in the following substances: Lemon, curd, tamarind and vinegar. (Page 11)
The acid present in the following substances are as follows:
- Lemon- Citric acids
- Curd- Lactic acid
- Tamarind- Tartaric acid
- Vinegar- Acetic acid
Question 2: If litmus is not available, are there some other natural substances that can serve as acid-base indicators? (Page 11)
Yes, if litmus is not available then there are some natural substances like turmeric, red cabbage juice, red rose extract and hibiscus which can be used as natural acid-base indicators.
Question 3: Can gardeners alter the colour of hydrangea flowers by adjusting the acidic or basic natural of the soil? (Page 14)
Yes, gardeners can change the colour of hydrangea flowers by adjusting the acidity or basicity (pH) of the soil.
- In acidic soil (pH below 6): the flowers usually turn blue.
- In neutral or basic (alkaline) soil (pH above 7): the flowers turn pink.
This happens because the availability of aluminium ions in the soil changes with pH. In acidic soil, aluminium is more available and gives the flowers a blue colour, while in basic soil, aluminium is less available, resulting in pink flowers.
Question 4: Can turmeric paper be used as an indicator for acidic substances? (Page 15)
No, turmeric paper cannot be used to test acidic substances because it does not change colour in acids.
Question 5: Are there any substances whose odours change on adding acidic or basic substances? (Page 16)
Yes, there are some substances whose odour changes in an acidic or basic medium. These are called as olfactory indicators.
Question 6: What happens when acidic substances mix with basic substances? (Page 17)
When an acidic substance mixes with a basic substance, they react with each other and neutralize the effects of both. This reaction is called a neutralization reaction.
- The acid and base combine to form salt and water.
- The resulting mixture is usually less acidic or less basic than the original substances.
Example: When lemon juice (acid) is mixed with baking soda (base), they react to form salt, water, and carbon dioxide gas (which causes fizzing).
Question 7: What remedies do people use to treat ant bites in your region? (Page 18)
When an ant bites, it injects a small amount of acid into the skin, which causes pain and irritation. People often use simple home remedies to treat ant bites, such as:
- Applying baking soda paste: Mix baking soda with a little water and apply it to the affected area. Baking soda is basic and helps neutralize the acid from the ant bite, reducing pain and itching.
- Using calamine lotion or aloe vera gel: These give a cooling effect and help soothe the skin.
- Rinsing with cold water or applying an ice pack: This helps reduce swelling and burning.
- Using toothpaste or honey: These can help calm irritation and prevent infection.
Question 8: If the factory waste is acidic in nature, what could be done to save the fish In the lake? (Page 19)
The factory waste can be neutralised by adding basic substances before releasing into the lake.
Question 9: Can you explain why the words 'Welcome to the Wonderful World of Science' appeared on Ashwin and Keerthi's paper sheets when the liquid was sprayed on them?
One possibility was that they could be using a turmeric solution for the spraying liquid and a soap solution for writing on the paper.
Let Us Enhance Our Learning : Exercise Questions
Question 1: A solution turns the red litmus paper to blue. Excess addition of which of the following solution would
reverse the change?
(i) Lime water (ii) Baking soda
(iii) Vinegar (iv) Common salt solution
(iii) Vinegar - (Vinegar is acidic in nature and acids turn blue litmus paper back to red.)
Question 2: You are provided with three unknown solutions labelled A, B, and C, but you do not know which of these are acidic, basic, or neutral. Upon adding a few drops of red litmus solution to solution A, it turns blue. When a few drops of turmeric solution are added to solution B, it turns red. Finally, after adding a few drops of red rose extract to solution C, it turns green. Based on the observations, which of the following is the correct sequence for the nature of solutions A, B, and C?
(i) Acidic, acidic, and acidic
(ii) Neutral, basic, and basic
(iii) Basic, basic, and acidic
(iv) Basic, basic, and basic
(iv) Basic, basic, and basic
Explanation : All three tests show that the solutions A, B, and Care basic in nature as the colour of the red litmus turns blue, turmeric turns red, and rose extract turns green in basic solutions.
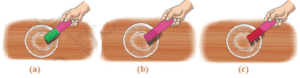
Question 3: Observe and analyse Figs. (a), (b) and (c), in which red rose extract paper strips are used. Label the nature of solutions present in each of the containers.
Since, red rose extract gives red colour in acidic solutions, green colour in basic solutions and remain same in colour in neutral solution.
Therefore, the solution in
Fig. (a) is basic,
Fig. (b) is neutral
Fig. (c) is acidic.
Question 4: A liquid sample from the laboratory was tested using various indicators:
| Indicator | Red litmus | Blue litmus | Turmeric |
| Change | No change | Turned red | No change in colour |
Based on the tests, identify the acidic or basic nature of the liquid and justify your answer.
Since, acid turns blue litmus to red and the colour of turmeric solution remains same in acidic solution, therefore, the given liquid sample is acidic in nature.
Question 5: Manya is blindfolded. She is given two unknown solutions to test and determine whether they are acidic or basic. Which indicator should Manya use to test the solutions and why?
Manya should use an olfactory indicator like onion or vanilla extract, because she cannot see, and these indicators show a change in smell with acids or bases, which she can easily detect by using her sense of smell.
Question 6: Could you suggest various materials which can be used for writing the message on the white sheet of paper (given at the beginning of the chapter) and what could be in the spray bottle? Make a table of various possible combinations and the colour of the writing obtained.
The various possible combinations are as follows:
| Writing Materials | Spray Bottle Contains | Colour of Writing Obtained |
| Turmeric paste | Soap solution | Re |
| Lemon juice | Red rose extract | Red |
| Baking soda solution | Red rose extract | Green |
Question 7: Grape juice was mixed with red rose extract; the mixture got a tint of red colour. What will happen if baking soda is added to this mixture? Justify your answer.
Red rose extract acts as a natural acid-base indicator. It turns red in acidic solutions and green in basic solutions.
- Grape juice is slightly acidic, so when mixed with red rose extract, the solution acquires a reddish tint.
- Baking soda is a mild base. When added to the mixture, it neutralizes the acid and increases the pH, making the solution basic.
- As a result, the red rose extract responds to the basic environment by changing its color from red to green.
Conclusion: Adding baking soda to the mixture will turn the red-colored solution green, indicating a shift from acidic to basic nature.
Question 8: Keerthi wrote a secret message to her grandmother on her birthday using orange juice. Can you assist her grandmother in revealing the message? Which indicator would you use to make it visible?
- Orange juice is acidic. Red rose extract is an indicator that turns red in acidic solutions.
- When the grandmother sprays or applies the red rose extract on the paper then the message written with orange juice will turn red and making it visible.
Question 9: How can natural indicators be prepared? Explain by giving an example.
Natural indicators can be prepared by extracting pigments from vegetables, fruits or flowers, such as beetroot, purple cabbage, turmeric, Indian blackberry (jamun) and red hibiscus (gudhal) flower.
Example : Red Cabbage Indicator :
Step 1: Chop red cabbage leaves.
Step 2: Boil them in water for 10–15 minutes.
Step 3: Cool and strain the purple-colored liquid.
Step 4: Use this extract to test solutions:
- Acidic solution → turns red or pink
- Neutral solution → remains purple
- Basic solution → turns green or yellow
Question 10: Three liquids are given to you. One is vinegar, another is a baking soda solution, and the third is a sugar solution. Can you identify them only using turmeric paper? Explain.
We will put a drop each of vinegar, baking soda solution, and sugar solution on the turmeric indicator paper, one by one.
- The liquid that changes the yellow colour of turmeric paper to red is basic in nature, hence it is the baking soda solution.
- Now, the red turmeric paper (made by the base) can be used to test the remaining two liquids.
When we put one drop each of the remaining liquids (vinegar and sugar solution) on the red turmeric paper:
- The drop of vinegar turns the red turmeric paper back to yellow, because vinegar is acidic and neutralises the basic effect of baking soda.
- The sugar solution causes no colour change, as it is neutral.
Question 11: The extract of red rose turns the liquid X to green. What will the nature of liquid X be? What will happen when excess of amla juice is added to liquid X?
- Since, red rose extract gives red colour in acidic solutions and green colour in basic solutions, therefore, the liquid X is basic in nature.
- When excess amla juice (which is acidic) is added to liquid X then the solution will become acidic and the red rose extract will turn red.
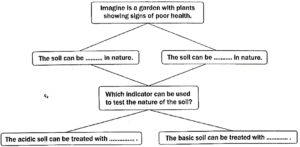
Question 12: Observe and analyse the information given in the following flowchart. Complete the missing information.
(i) The soil can be acidic in nature.
(ii) The soil can be basic in nature.
(iii) The indicator used to test the nature of soil is acid-base indicator (like blue and red litmus paper).
(iv) The acidic soil can be treated with lime.
(v) The basic soil can be treated with organic matter.
Key Features of Kitabcd Exam Master :
|
Click on below links to get PDF from store
PDF : Chapter-2-Exploring Substances: Acidic, Basic, and Neutral– Notes
PDF : Chapter-2-Exploring Substances: Acidic, Basic, and Neutral– Exam Master
Main Page : NCERT-Class-7-Science (Curiosity) – All chapters notes, solutions, videos, test, pdf.
Next Chapter : Chapter-1- The Ever-Evolving World of Science – Online Solutions
Next Chapter : Chapter-3-Electricity: Circuits and their Components – Online Solutions
We reply to valid query.