Elements of Groups 16, 17, and 18
Maharashtra Board-Class-12-Chemistry-Chapter-7
Notes-Part-1
Question 1. Select appropriate answers for the following.
(i) Which of the following has highest electron gain enthalpy ?
(A) Fluorine
(B) Chlorine
(C) Bromine
(D) Iodine
(B) Chlorine
Reason : Chlorine is larger in size and has a lower electronegativity value than fluorine. As a result, it readily accepts an electron and has the highest electron gain enthalpy value.
(ii) Hydrides of group 16 are weakly acidic. The correct order of acidity is
(A) H2O > H2S > H2Se > H2Te
(B) H2Te > H2O > H2S > H2Se
(C) H2Te > H2Se > H2S > H2O
(D) H2Te > H2Se > H2O > H2S
(C) H2Te > H2Se > H2S > H2O
(iii) Which of the following element does not show oxidation state of +4 ?
(A) O
(B) S
(C) Se
(D) Te
(A) O
(iv) HI acid when heated with conc. H2SO4 forms
(A) HIO3
(B) KIO3
(C) I2
(D) KI
(C) I2
(v) Ozone layer is depleted by
(A) NO
(B) NO2
(C) NO3
(D) N2O5
(A) NO
(vi) Which of the following occurs in liquid state at room temperature ?
(A) HIO3
(B) HBr
(C) HCl
(D) HF
(D) HF
(vii) In pyrosulfurous acid oxidation state of sulfur is
(A) Only +2
(B) Only +4
(C) +2 and +6
(D) Only +6
(D) Only +6
(viii) Stability of interhalogen compounds follows the order
(A) BrF > IBr > ICl > ClF > BrCl
(B) IBr > BeF > ICl > ClF > BrCl
(C) ClF > ICl > IBr > BrCl > BrF
(D) ICl > ClF > BrCl > IBr > BrF
(C) ClF > ICl > IBr > BrCl > BrF
(ix) BrCl reacts with water to form
(A) HBr
(B) Br2 + Cl2
(C) HOBr
(D) HOBr + HCl
(D) HOBr + HCl
(x) Chlorine reacts with excess of fluorine to form.
(A) ClF
(B) ClF3
(C) ClF2
(D) Cl2F3
(B) ClF3
(xi) In interhalogen compounds, which of the following halogens is never the central atom.
(A) I
(B) Cl
(C) Br
(D) F
(D) F
(xii) Which of the following has one lone pair of electrons ?
(A) IF3
(B) ICl
(C) IF5
(D) ClF3
(C) IF5
(xiii) In which of the following pairs, molecules are paired with their correct shapes ?
(A) [I3] : bent
(B) BrF5 : trigonal bipyramid
(C) ClF3 : trigonal planar
(D) [BrF4] : square planar
(D) [BrF4] : square planar
(xiv) Among the known interhalogen compounds, the maximum number of atoms is
(A) 3
(B) 6
(C) 7
(D) 8
(D) 8
Question 2. Answer the following.
(i) Write the order of the thermal stability of the hydrides of group 16 elements.
The decreasing order of thermal stability: H2O > H2S > H2Se > H2Te
(ii) What is the oxidation state of Te in TeO3 ?
Oxygen (O) typically has an oxidation state of −2.
In TeO₃, there are three oxygen atoms, contributing a total of −6.
Therefore, to balance this, tellurium must have an oxidation state of +6.
Oxidation state of Te in TeO3 is +6
(iii) Name two gases which deplete ozone layer.
Two gases deplete ozone layer :
(i) Nitrogen oxide (NO) released from exhaust systems of car or supersonic jet aeroplanes
(ii) Chlorofluorocarbons (Freons) used in aerosol sprays and refrigerators
(iv) Give two uses of ClO2
- ClO2 is used as a bleaching agent for paper pulp and textiles.
- It is also used in water treatment.
(v) What is the action of bromine on magnesium metal ?
Bromine reacts instantly with magnesium metal to give magnesium bromide.
Mg(s) + Br2(l) → MgBr2(s)
(vi) Write the names of allotropic forms of selenium.
Selenium has two allotropic forms as follows :
- Red (non-metallic) form
- Grey (metallic) form
(vii) What is the oxidation state of S in H2SO4.
(Oxidation number of H) + (Oxidation number of S) + (Oxidation number of O) = 0
2 x (+1) + (Oxidation number of S) + 4 x (-2) = 0
Oxidation number of S + 2 - 8 = 0
Hence, oxidation number of 'S' in H2SO4 = +6
(viii) The pKa values of HCl is −7.0 and that of HI is −10.0. Which is the stronger acid ?
For HCl, pKa = − 7.0, hence its dissociation constant is, Ka = 1 x 10−7.
For HI pKa = − 10.0, hence its dissociation constant is Ka = 1 x 10−10.
Hence HCl dissociates more than HI.
Therefore HCl is a stronger acid than HI.
(ix) Give one example showing reducing property of ozone.
Ozone as a reducing agent : Ozone reduces peroxides (O1-) to oxides (O2-).
Ozone reduces barium peroxide, BaO2 to barium oxide, BaO.
BaO2 + O3 → BaO + 2O2
Ozone reduces hydrogen peroxide, H2O2 to water, H2O.
H2O2 + O3 → H2O + 2O2
(x) Write the reaction of conc. H2SO4 with sugar.
Concentrated sulphuric acid when added to sugar, it is dehydrated giving carbon.
C12H22O11 \(\underrightarrow{conc.\,H_2SO_4}\) 12C + 11H2O
The carbon that is left behind is called sugar charcoal and the process is called charring.
(xi) Give two uses of chlorine.
Chlorine is used :
- for bleaching wood pulp required for the manufacture of paper and rayon, cotton and textiles.
- in the manufacture of organic compounds like CHÇl3, CCl4, DDT, dyes and drugs.
(xii) Complete the following.
(1) ICl3 + H2O ........ + ...... + ICl
(2) I2 + KClO3 ....... + KIO3
(3) BrCl + H2O ....... + HCl
(4) Cl2 + ClF3 ........
(5) H2C = CH2 + ICl .......
(6) XeF4 + SiO2 ....... + SiF4
(7) XeF6 + 6H2O ........ + HF
(8) XeOF4 + H2O ....... + HF
(1) 2ICl3 + 3H2O → 5HCl + HlO3 + ICl
(2) I2 + KClO3 → ICl + KIO3
(3) BrCl + H2O → HOBr + HCl
(4) Cl2 + ClF3 → 3ClF
(5) CH2 = CH2 + ICL → CH2l-CH2Cl
(6) 2XeF6 + SiO2 → 2XeOF4 + SiF4
(7) XeF6 + 3H2O → XeO3 + 6HF
(8) XeOF4 + H2O → XeO2F2 + 2HF
(xiii) Match the following
A B
XeOF2 Xenon trioxydifluoride
XeO2F2 Xenon monooxydifluoride
XeO3F2 Xenon dioxytetrafluoride
XeO2F4 Xenon dioxydifluoride
XeOF2 - Xenon monooxydifluoride
XeO2F2 - Xenon dioxydifluoride
XeO3F2 - Xenon trioxydifluoride
XeO2F4 - Xenon dioxytetrafluoride
(xiv) What is the oxidation state of xenon in the following compounds.
XeOF4, XeO3, XeF6, XeF4, XeF2.
Compounds |
Oxidation state of xenon |
| XeOF4 | +6 |
| XeO3 | +6 |
| XeF6 | +6 |
| XeF4 | +4 |
| XeF2 | +2 |
Question 3. Answer the following.
(i) The first ionisation enthalpies of S, Cl and Ar are 1000, 1256 and 1520 kJ/mol−1, respectively. Explain the observed trend.
- The atomic number increases as, 16S < 17Cl < 18
- Due to decrease in atomic size and increase in effective nuclear charge, Cl binds valence electrons strongly.
- Hence ionisation enthalpy of Cl (1256 kJ mol−1) is higher than that of S(1000 kJ mol−1)
- Ar has electronic configuration 3s23p6. Since all electrons are paired and octet is complete, it has the highest ionisation enthalpy, (1520 kJ mol−1)
(ii) “Acidic character of hydrides of group 16 elements increases from H2O to H2Te” Explain.
- The thermal stability of the hydrides of group 16 elements decreases from H2O to H2 This is because the bond dissociation enthalpy of the H-E bond decreases down the group.
- Thus, the acidic character increases from H2O to H2
(iii) How is dioxygen prepared in laboratory from KClO3 ?
Laboratory methods : By heating chlorates, nitrates and permanganates :
Potassium chlorate in the presence of manganese dioxide on heating decomposes to form potassium chloride and oxygen.
2KClO3 \(\underrightarrow{ΔMnO_2}\) 2KCl + 3O2(g)
(iv) What happens when
(a) Lead sulfide reacts with ozone (O3).
(b) Nitric oxide reacts with ozone.
(i) It oxidises lead sulphide (PbS) to lead sulphate (PbSO4) changing oxidation state of S from -2 to +6.
PbS(s) + 4O3(g) → PbSO4(s) + 4O2(g)
(ii) Ozone oxidises nitrogen oxide to nitrogen dioxide.
NO(g) + O3(g) → NO2(g) + O2(g)
(v) Give two chemical reactions to explain oxidizing property of concentrated H2SO4.
(i) Hot and concentrated H2SO4 acts as an oxidizing agent, since it gives nascent oxygen on heating.
H2SO4 \(\underrightarrow{Δ}\) H2O + SO2 + [O]
(conc.)
(ii) It oxidises metals and non-metals. For example,
Cu(s) + 2H2SO4 → CuSO4 + 2H2O + SO2
(metal) (conc.)
C + 2H2SO4 → CO2 +2SO2 +2H2O
(non-metal)
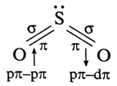
(vi) Discuss the structure of sulfure dioxide.
- SO2 molecule has a bent V shaped structure with S-O-S bond angle 119.5° and bond dissociation enthalpy is 297 kJ mol-1.
- Sulphur in SO2 is sp2 hybridised forming three hybrid orbitals. Due to lone pair electrons, bond angle is reduced from 120° to 119.5°.
- In SO2, each oxygen atom is bonded to sulphur by a σ and a π
- σ bonds between S and O are formed by sp2-p overlapping.
- One of π bonds is formed by pπ -pπ overlapping while other n bond is formed by pπ -dπ
- Due to resonance both the bonds are identical having observed bond length 143 pm due to resonance,

Partially filled 3p1z and 3d1 orbitals overlap to form π bonds with oxygen atoms.
(vii) Fluorine shows only −1 oxidation state while other halogens show −1, +1, +3, +5 and +7 oxidation states. Explain.
- The fluorine atom has no d-orbitals in its valence shell and therefore, cannot expand its octet. Thus, fluorine is the most electronegative exhibit -1 oxidation state only.
- Cl, Br, and I exhibit -1, +1, +3, +5, and +7 oxidation states. This is because they are less electronegative than F and possess empty d-orbitals in the valence shell and therefore, can expand the octet.
(viii) What is the action of chlorine on the following
(a) Fe (b) Excess of NH3
(a) Chlorine reacts with Fe to give ferric chloride.
2Fe + 3Cl2 → 2FeCl3
(b) Chlorine reacts with the excess of ammonia to form ammonium chloride, NH4Cl and nitrogen.
8NH3 + 3Cl2 → 6NH4Cl + N2
(excess)
(ix) How is hydrogen chloride prepared from sodium chloride ?
- In the laboratory, hydrogen chloride is prepared by heating sodium chloride (common salt) with concentrated sulfuric acid.
NaCl + H2SO4 \(\underrightarrow{420K}\) NaHSO4 + HCl
NaHSO4 + NaCl \(\underrightarrow{420K}\) Na2SO4 + HCl
- HCl gas can be dried by passing it through concentrated sulfuric acid and then collected by upward displacement of air.
(x) Draw structures of XeF6, XeO3, XeOF4, XeF2.

(xi) What are inter−halogen compounds ? Give two examples.
Interhalogen compounds : Compounds formed by the combination of atoms of two different halogens are called interhalogen compounds.
- In an interhalogen compound, of the two halogen atoms, one atom is more electropositive than the other.
- The interhalogen compound is regarded as the halide of the more electropositive halogen. For example, ClF, BrF3, ICl
(xii) What is the action of hydrochloric acid on the following ?
(a) NH3 (b) Na2CO3
Hydrochloric acid reacts with ammonia to give white fumes of ammonium chloride.
NH3 + HCl → NH4Cl
Hydrochloric acid reacts with sodium carbonate to give sodium chloride, water with the liberation of carbon dioxide gas.
Na2CO3 + 2HCl → 2NaCl +H2O + CO2
(xiii) Give two uses of HCl.
Uses of hydrogen chloride (hydrochloric acid) :
- in the manufacture of chlorine and ammonium chloride,
- to manufacture glucose from corn, starch
- to manufacture dye
- in medicine and galvanising
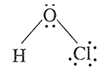
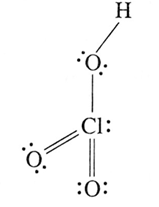
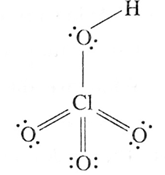
(xiv) Write the names and structural formulae of oxoacids of chlorine.
(i) Hypochlorous acid, HClO :

(ii) Chlorous acid, HClO2 :
(iii) Chloric acid, HClO3 :
(iv) Perchloric acid, HClO4 :
(xv) What happens when
(a) Cl2 reacts with F2 in equal volume at 437 K.
(b) Br2 reacts with excess of F2.
(a) Cl2 reacts with F2 in equal volumes at 437 K to give chlorine monofluoride ClF.
Cl2 + F2 \(\underrightarrow{437K}\) 2ClF
(b) Br2 reacts with excess of F2 to give bromine trifluoride BrF3.
Br2 + 3F2 \(\underrightarrow{Δ}\) 2BrF3.
(excess)
(xvi) How are xenon fluorides XeF2, XeF4 and XeF6 obtained ? Give suitable reactions.
Preparation of Xenon Fluorides : Xenon fluorides are generally prepared by direct reaction of xenon and fluorine in different ratios and conditions, such as temperature, electric discharge and photochemical reaction.
(i) Preparation of XeF2 :
Xe + F2 \(\frac{sealed\,\,Ni\,\,tube}{400^0C}\)-> XeF2
(ii) Preparation of XeF4 :
Xe + F2 \(\frac{Ni\,\,tube\,\,400^0C}{5-6\,atoms}\)-> XeF4
1 : 5
Xe + 2F2 \(\frac{electric\,\,discharge}{-80^0C}\)-> XeF4
(ii) Preparation of XeF6 :
Xe + 3F2 \(\frac{electric\,\,discharge}{low\,\,temp.}-->\) XeF6
(xvii) How are XeO3 and XeOF4 prepared ?
Preparation of XeO3 : Xenon trioxide ( XeO3) is prepared by the hydrolysis of XeF4 or XeF6
(i) By hydrolysis of XeF4 :
3XeF4 + 6H2O → 2Xe + XeO3 + 12HF + O2
(ii) By hydrolysis of XeF6 :
XeF6 + 3H2O → XeO3 + 6HF
Preparation of XeOF4 : Xenon oxytetrafluoride (XeOF4) is prepared by the partial hydrolysis of XeF6.
XeF + H2O → XeOF4 + 2HF
(xviii) Give two uses of neon and argon.
Uses of neon (Ne) :
- Neon is used in the production of neon discharge lamps and signs by filling Ne in glass discharge tubes.
- Neon signs are visible from a long distance and also have high penetrating power in mist or fog.
- A mixture of neon and helium is used in voltage stabilizers and current rectifiers.
- Neon is also used in the production of lasers and fluorescent tubes.
Uses of argon (Ar) :
- Argon is used to fill fluorescent tubes and radio valves.
- It is used to provide inert atmosphere for welding and production of steel.
- It is used along with neon in neon sign lamps to obtain different colours.
- A mixture of 85% Ar and 15% N2 is used in electric bulbs to enhance the life of the filament.
(xix) Describe the structure of Ozone. Give two uses of ozone.
Ozone has molecular formula O3. The lewis dot and dash structures for O3 are :
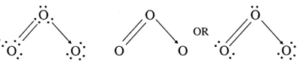
- Infrared and electron diffraction spectra show that O3 molecule is angular with O-O-O bond angle 117°.
- Both O-O bonds are identical having bond length 128 pm which is intermediate between single and double bonds.
- This is explained by considering resonating structures and resonance hybrid.

Uses of Ozone :
- Sterilizes drinking water by oxidizing germs and bacteria.
- Used as bleaching agent for ivory, oils, starch, wax, and delicate fabrics.
- Purifies air in crowded places like cinema halls, railways, tunnels.
- In industry, used in manufacturing synthetic camphor, potassium permanganate.
(xx) Explain the trend in following atomic properties of group 16 elements.
(i) Atomic radii
- Group 16 elements have smaller atomic and ionic radii due to higher nuclear charge compared to group 15 elements.
- As the group moves down from oxygen to polonium, the atomic and ionic radii increase due to the addition of a new shell.
- The atomic radii increases in the order 0 < S < Se < Te < Po
(ii) Ionisation enthalpy
- The ionisation enthalpy of group 16 elements has quite high values.
- Ionisation enthalpy decreases down the group from oxygen to polonium. This is due to the increase in atomic volume down the group.
- The first ionisation enthalpy of the lighter elements of group 16 (O, S, Se) have lower values than those of group 15 elements in the corresponding periods. This is due to difference in their electronic configurations.
Group 15 : (valence shell) ns2 npx1 npy1 npz1
Group 16 : (valence shell) ns2 npx2 npy1 npz1
- Group 15 elements have extra stability of half filled and more symmetrical orbitals, while group 16 elements acquire extra stability by losing one of paired electrons from npx −orbital forming half filled p−orbitals.
Hence group 16 elements have lower first ionisation enthalpy than group 15 elements.
(iii) Electronegativity.
- The electronegativity values of group 16 elements have higher values than corresponding group 15 elements in the same periods.
- Oxygen is the second most electronegative elements after fluorine.(O = 3.5, F = 4)
- On moving down the group electronegativity decreases from oxygen to polonium.
- On moving down the group atomic size increases, hence nuclear attraction decreases, therefore electro−negativity decreases.
Question 4. Answer the following.
(i) Distinguish between rhombic sulfur and monoclinic sulfur.
| Rhombic sulfur | Monoclinic sulfur |
| It is a pale yellow coloured solid. | It is bright yellow solid |
| It forms orthorhombic crystals | It forms needle-shaped monoclinic crystals |
| Its melting point is 385.8 K. | Its melting point is 393 K. |
| Its density, 2.069 g / cm3 | Its Density: 1.989g / cm3 |
| Insoluble in water, but soluble in CS2 | Soluble in CS2 |
| It is stable below 369 K and transforms to ∝-sulphur above this temperature. | It is stable above 369 K and transforms into β -sulphur below this temperature. |
| It exists as Sg molecules with a structure of a puckered ring. | It exists as Sg molecules with a structure of a puckered ring. |
| It is obtained by the evaporation of roll sulphur in CS2 | It is prepared by melting rhombic Sulphur and cooling it till a crust is formed. Two holes are pierced in the
crust and the remaining liquid is poured to obtain needle-shaped crystals of monoclinic sulphur (β-sulphur). |
(ii) Give two reactions showing oxidizing property of concentrated H2SO4.
(i) Hot and concentrated H2SO4 acts as an oxidizing agent, since it gives nascent oxygen on heating.
H2SO4 \(\underrightarrow{Δ}\) H2O + SO2 + [O]
(conc.)
(ii) It oxidises metals and non-metals. For example,
Cu(s) + 2H2SO4 → CuSO4 + 2H2O + SO2
(metal) (conc.)
C + 2H2SO4 → CO2 +2SO2 +2H2O
(non-metal)
(iii) How is SO2 prepared in laboratory from sodium sulfite? Give two physical properties of SO2.
(i) Sodium sulphite on treating with dilute H2SO4 forms SO2
Na2SO3 + H2SO4(aq) → Na2SO4 + H2O(l) + SO2(g)
(ii) Sodium sulphite, Na2SO3 on reaction with dilute hydrochloric acid solution forms SO2.
Na2SO3(aq) + 2HCl(aq) → 2NaCl(ag) + H2O(l) + SO2(g)
Physical properties of SO2 :
- It is a colourless gas with a pungent smell.
- It is highly soluble in water and forms sulphurous acid, H2SO3
SO2(g) + H2O(l) → H2SO3(aq)
- It is poisonous in nature.
- At room temperature, it liquefies at 2 atmospheres. It has boiling point 263 K.
.
(iv) Describe the manufacturing of H2SO4 by contact process.
Sulfuric acid is manufactured by Contact process, which involves the following three steps.
(i) Preparation of SO2 : Sulfur or sulfide ore (iron pyrites) on burning or roasting in air produces sulfur dioxide.
S(s) + O2(g) \(\underrightarrow{Δ}\) SO2(g)
4FeS2(s) + 11O2 (g) \(\underrightarrow{Δ}\) 2Fe2O3(s) + 8SO2 (g)
(ii) Oxidation of SO2 to SO3 : Sulfur dioxide is oxidised catalytically with oxygen to sulfur trioxide, in the presence of V2O5 catalyst.
2SO2(g) + O2 \(\underrightarrow{V_2O_5}\) 2SO3(g)
The reaction is exothermic and reversible and the forward reaction leads to decrease in volume. Therefore low temperature (720K) and high pressure (2 bar) are favourable conditions for maximum yield of SO3.
(iii) Dissolution of SO3 : Sulfur trioxide gas (from the catalytic converter) is absorbed in concentrated H2SO4 to produce oleum.
Dilution of oleum with water gives sulfuric acid of desired concentration.
SO3(g) + H2SO4 → H2S2O7 (oleum)
H2S2O7 + H2O → 2H2SO4
The sulfuric acid obtained by contact process is 96 - 98 % pure.
Flow diagram for manufacture of Sulfuric acid

Chapter-7-Elements of Groups 16, 17, and 18-Text Book-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Notes-PDF
Chapter-7-Elements of Groups 16, 17, and 18- Solution-PDF
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter-6-Chemical Kinetics – Online Solutions
Next Chapter : Chapter-8-Transition and Inner transition Elements – Online Solutions
We reply to valid query.