Introduction to Polymer Chemistry
Maharashtra Board-Class-12-Chemistry-Chapter-15
Solutions
Question 1. Choose the correct option from the given alternatives.
(i) Nylon fibres are ----
(A) Semisynthetic fibres
(B) Polyamide fibres
(C) Polyester fibres
(D) Cellulose fibres
(B) Polyamide fibres
(ii) Which of the following is naturally occurring polymer ?
(A) Telfon
(B) Polyethylene
(C) PVC
(D) Protein
(D) Protein
(iii) Silk is a kind of ---- fibre
(A) Semisynthetic
(B) Synthetic
(C) Animal
(D) Vegetable
(C) Animal
(iv) Dacron is another name of ----
(A) Nylon 6
(B) Orlon
(C) Novolac
(D) Terylene
(D) Terylene
(v) Which of the following is made up of polyamides ?
(A) Dacron
(B) Rayon
(C) Nylon
(D) Jute
(C) Nylon
(vi) The number of carbon atoms present in the ring of e - caprolactam is
(A) Five
(B) Two
(C) Seven
(D) Six
(D) Six
(vii) Terylene is ----
(A) Polyamide fibre
(B) Polyester fibre
(C) Vegetable fibre
(D) Protein fibre
(B) Polyester fibre
(viii) PET is formed by ----
(A) Addition
(B) Condensation
(C) Alkylation
(D) Hydration
(B) Condensation
(ix) Chemically pure cotton is ----
(A) Acetate rayon
(B) Viscose rayon
(C) Cellulose nitrate
(D) Cellulose
(D) Cellulose
(x) Teflon is chemically inert, due to presence of ...........
(A) C-H bond
(B) C-F bond
(C) H- bond
(D) C=C bond
(B) C-F bond
Question 2. Answer the following in one sentence each.
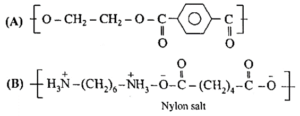
(i) Identify 'A' and 'B' in the following reaction ----
(a)
![]()
(b) H2N-(CH2)6-NH2 + HOOC-(CH2)4COOH \(\frac{N_2}{533K}>\) ‘B’
(ii) Complete the following statements
(a) Caprolactam is used to prepare--------
(b) Novolak is a copolymer of ------ -- and ---------
(c) Terylene is ----------polymer of terephthalic acid and ethylene glycol.
(d) Benzoyl peroxide used in addtion polymerisation acts as ----------
(e) Polyethene consists of polymerised ----------
(a) Caprolactam is used to prepare Nylon-6
(b) Novolak is a copolymer of Phenol and formaldehyde.
(c) Terylene is polyester polymer of terephthalic acid and ethylene glycol.
(d) Benzoyl peroxide used in addtion polymerisation acts as initiator (catalyst).
(e) Polyethene consists of polymerised linear or branched chain.
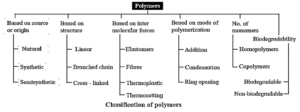
(iii) Draw the flow chart diagram to show classification of polymers based on type of polymerisation.
(iv) Write examples of Addition polymers and condensation polymers.
Addition polymers : Polyvinyl chloride, polythene
Condensation polymers : Bakelite, terylene, Nylon-66
(v) Name some chain growth polymers.
Chain growth polymers : Polythene, polyacrylonitrile and polyvinyl chloride.
(vi) Define the terms :
(1) Monomer
Monomer is a small and simple molecule and has a capacity to form two chemical bonds with other monomers.
Examples : Ethene, Propylene.
(2) Vulcanisation
Vulcanisation is the technique of introducing a network of cross links into an elastomer. It can also be defined as the process of heating natural rubber with sulphur to increase the tensile strength, toughness, and elasticity of natural rubber.
(3) Synthetic fibres
The man-made fibres prepared by polymerization of one monomer or copolymerization of two or more monomers are called synthetic fibres.
(vii) What type of intermolecular force leads to high density polymer ?
High density polymers have low degree of branching along the hydrocarbon chain. The molecules are closely packed together during crystallization. This closer packing means that the van der Waals attraction between the chains are greater and so the plastic (high density polymer) is stronger and has a melting point.
(viii) Give one example each of copolymer and homopolymer.
Homopolymer : PVC, Nylon-6
Copolymer : Terylene, Buna-S
(ix) Identify Thermoplastic and Thermosetting Plastics from the following --
(1) PET
(2) Urea formaldehyde resin
(3) Polythene
(4) Phenol formaldehyde
Thermoplastic plastics : PET, Polythene
Thermosetting plastics : Urea formaldehyde resin, Phenol formaldehyde.
Question 3. Answer the following.
(i) Write the names of classes of polymers formed according to intermolecular forces and describe briefly their structural characteristics.
Mechanical properties of polymers such as tensile strength, toughness, elasticity differ widely depending upon the intermolecular forces. Polymers are classified into various categories on the basis of intermolecular forces as follows.
(i) Elastomers :
- Weak van der Waals type of intermolecular forces of attraction between the polymer chains are observed in elastomers, which permit the polymer to be stretched.
- When polymer is stretched, the polymer chain stretches and when the strain is relieved the chain returns to its original position. Thus, polymers have elastic character like rubber are called elastomers.
- Elastomers are soft and stretchy and used in making rubber bands.
- Examples : Neoprene, vulcanized rubber, buna-S, buna-N.
(ii) Fibres :
- Polymeric solids which form threads are called fibres.
- The fibres possess high tensile strength which is a property to have resistance to breaking under tension.
- This characteristic is due to the strong intermolecular forces like hydrogen bonding and strong dipole-dipole forces.
- Due to these strong intermolecular forces the fibres are crystalline in nature.
- Examples : Polyamides (nylon 6, 6), polyesters (terylene), etc.
(iii) Thermoplastic polymers :
- These polymer possess moderately strong intermolecular forces of attraction between those of elastomers and fibres.
- These polymers are called thermoplastic because they become soft on heating and hard on cooling.
- They are either linear or branched chain polymers. They can be remoulded and recycled.
- Examples : Polyethene, PVC, polystyrene, PET.
(iv) Thermosetting polymers :
- These polymers are cross linked or branched molecules and are rigid polymers.
- During their formation they have property of being shaped on heating, but they get hardened while hot.
- Once hardened these become infusible, cannot be softened by heating and therefore, cannot be remoulded and recycled.
- This shows extensive cross linking by covalent bonds formed in the moulds during hardening/setting process while hot.
- Examples : Bakelite, urea formaldehyde resin, phenol formaldehyde.
(ii) Write reactions of formation of :
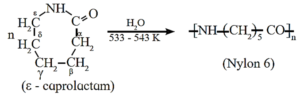
(a) Nylon 6
When epsilon (ε)-caprolactam is heated with water at high temperature it undergoes ring opening polymerization to give the polyamide polymer called nylon 6.
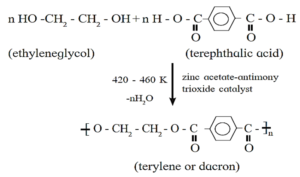
(b) Terylene
- Terylene is formed by the polymerization of terephthalic acid and ethylene glycol.
- Terylene is obtained by condensation polymerization of ethylene glycol and terephthalic acid in presence of catalyst zinc acetate and antimony trioxide at high temperature.
(iii) Write structure of natural rubber and neoprene rubber along with the name and structure of their monomers.
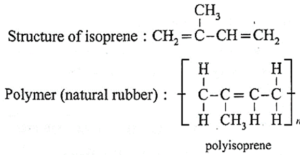
(a) Natural rubber :
Name : Polyisoprene
Structure of monomer
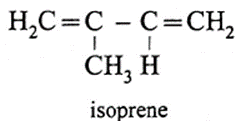
(b) Synthetic rubber :
Name : Neoprene
Structure of monomer
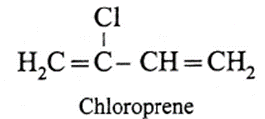
(iv) Name the polymer type in which following linkage is present.
![]()
In terylene or dacron polymer.
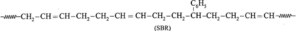
(v) Write structural formula of the following synthetic rubbers :
(a) SBR rubber
(b) Buna-N rubber
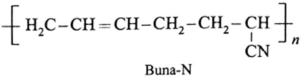
(c) Neoprene rubber
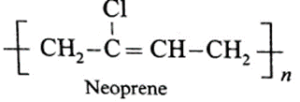
(vi) Match the following pairs :
| Name of polymer | Monomer |
| 1. Teflon | a) CH2=CH2 |
| 2. PVC | b) CF2=CF2 |
| 3. Polyester | c) CH2=CHC(I) |
| 4. Polythene | d) C6H5OH and HCHO |
| 5. Bakelite | e) Dicarboxylic acid and polyhydoxyglycol |
(1) Teflon - CF2= CF2
(2) PVC - CH2 = CHC(I)
(3) Polyester - Dicarboxylic acid and polyhydroxyglycol
(4) Polythene - CH2= CH2
(5) Bakelite - C6H5OH and HCHO
(vii) Draw the structures of polymers formed from the following monomers
(1) nHOOC-R-COOH + nHO-R'-OH
(2) H2N-(CH2)5-COOH
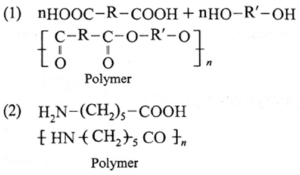
(viii) Name and draw structure of the repeating unit in natural rubber.
Repeating unit of natural rubber (Basic unit : isoprene)
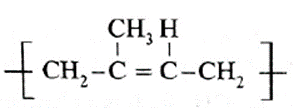
(ix) Classify the following polymers as natural and synthetic polymers
(a) Cellulose,
(b) Polystyrene,
(c) Terylene, (d) Starch,
(e) Protein
(f) Silicones,
(g) Orlon (Polyacrylonitrle),
(h) Phenol-formedehyde resins
Natural Polymers : (a) Cellulose , (d) Starch, (e) Protein
Synthetic Polymers : (b) Polystyrene, (c) Terylene , (f) Silicones, (g) Orlon (Polyacrylonitrle), (h) Phenol-formedehyde resins
(x) What are synthetic resins? Name some natural and synthetic resins.
Synthetic resins are artificially synthesised high molecular weight polymers. They are the basic raw material of plastic. The main properties of plastic depend on the synthetic resin it is made from.
Natural resins: Natural rubber, silk, wool, etc.
Examples of synthetic resins : Polyester resin, Phenolic resin, Alkyl resin, Polycarbonate resin, Silicone resin, Epoxy resin, Acrylic resin etc.
(xi) Distinguish between thermosetting and thermoplastic resins. Write example of both the classes.
| Thermosetting resins | Thermoplastic resins |
| They do not soften on heating. | They soften on heating and harden on cooling. |
| They cannot be remoulded or reshaped. | These can be remoulded or reshaped. |
| They possess extensive cross-linking formed by covalent bonds. | They possess moderately strong intermolecular forces that are intermediate between elastomers and fibres. |
| They are rigid polymers. | They are not rigid polymers. |
| e.g. Bakelite, urea-formaldehyde resins, etc. | e.g. PVC, polythene, polystyrene, etc. |
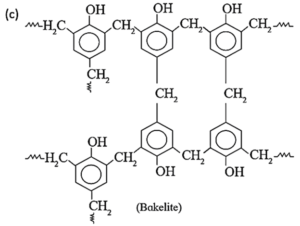
(xii) Write name and formula of raw material from which bakelite is made.
The raw material or monomers used to prepare bakelite are
(i) o-hydroxymethyl phenol :
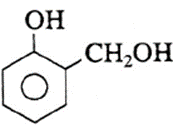
and
(ii) formaldehyde : (HCHO).
Question 4. Attempt the following :
(i) Identify condensation polymers and addition polymers from the following.
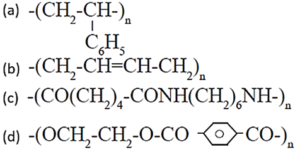
(a ) Addition polymer
(b) Addition polymer
(c) Condensation polymer
(d) condensation polymer
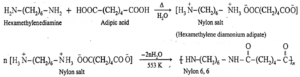
(ii) Write the chemical reactions involved in manufacture of Nylon 6,6
Nylon-6, 6 is a linear polyamide polymer formed by the condensation polymerisation reaction. The monomers used in the preparation of Nylon—6, 6 are :
- Adipic acid : HOOC—(CH2)4—COOH
- Hexamethylene diamine : H2N—(CH2)6—NH2
When equimolar aqueous solutions of adipic acid and hexamethylene diamine are mixed and heated, there is neutralization to form a nylon salt. During polymerisation at 553 K nylon salt loses a water molecule to form nylon 6, 6 polymer. Both monomers (hexamethylene diamine and adipic acid) contain six carbon atoms each, hence the polymer is termed as Nylon—6,6.
Properties : Nylon 6,6 is high molecular mass (12000-50000 u) linear condensation polymer. It possesses high tensile strength. It does not soak in water.
Uses : It is used for making sheets, bristles for brushes, surgical sutures, textile fabrics, etc.
(iii) Explain vulcanisation of rubber. Which vulcanizing agents are used for the following synthetic rubber.
(a) Neoprene (b) Buna-N
The process by which a network of cross links is introduced into an elastomer is called vulcanization.
Vulcanization enhances the properties of natural rubber like tensile strength, stiffness, elasticity, toughness etc.
Sulphur forms cross links between polyisoprene chains which results in improved properties of rubber.
(a) For neoprene vulcanizing agent is MgO.
(b) For Buna-N vulcanizing agent is sulphur.
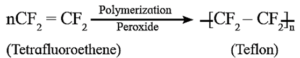
(iv) Write reactions involved in the formation of ---
(1) Teflon
- The monomer used in preparation of teflon is tetrafluoroethylene, (CF2=CF2) which is a gas at room temperature.
- Tetrafluoroethylene is polymerized by using free radical initiators such as hydrogen peroxide or ammonium persulphate at high pressure.
(2) Bakelite
Bakelite : The ortho and para substituted phenols can undergo polymerization to produce a cross linked polymer known as bakelite.
The raw material or monomers used to prepare bakelite are o-hydroxymethyl phenol and formaldehyde (HCHO).
Phenol and formaldehyde react in presence of acid or base catalyst to form thermosetting/moulding powder (novolac) in two stages.
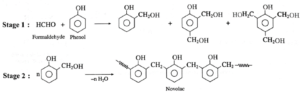
In the third stage, various articles are shaped from novolac by putting it in appropriate moulds and heating at high temperature (1380C to 1760C) and at high pressure forms rigid polymeric material called bakelite. Bakelite is insoluble and infusible and has high tensile strength.

(v) What is meant by LDP and HDP? Mention the basic difference between the same with suitable examples.
- LDP is low density polyethylene and HDP is high density polyethylene.
- LDP is a branched polymer with low density due to chains are loosely held and HDP is a linear polymer with density due to close packing.
- HDP is much stiffer than LDP and has high tensile strength and hardness.
- LDP is mainly used in preparation of pipes for agriculture, irrigation and domestic water line connections. HDP is used in manufacture of toys and other household articles like bucket, bottles, etc.
(vi) Write preparation, properties and uses of Teflon.
Teflon is polytetrafluoroethylene. It is an addition polymer of tetrafluoroethene.
The monomer used in preparation of Teflon is tetrafluoroethylene, (CF2=CF2) which is a gas at room temperature.
Tetrafluoroethylene is polymerized by using free radical initiators such as hydrogen peroxide or ammonium persulphate at high pressure.
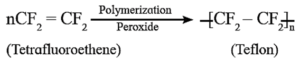
Properties :
- Teflon is tough, chemically inert and heat resistant and resists corrosive attacks of acids and bases.
- C—F bond is very difficult to break and remains unaffected by corrosive alkali, organic solvents.
Uses : Teflon is used in making blankets, carpets, non-stick cookware, oil seals, gaskets, etc.
(vii) Classify the following polymers as straight chain, branched chain and cross linked polymers.
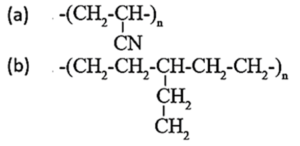
(a) Straight chain polymers.
(b) Branched chain polymers.
(c) Cross linked polymers.
Question 5. Answer the following.
(i) How is polythene manufactured ? Give their properties and uses.
Polythene is of two kinds, namely low density polythene (LDP) and high density polythene (HDP) .
(a) Low density polyethylene (LDP) : LDP is obtained by polymerization of ethylene under high pressure (1000-2000 atm) and temperature (350-570 K) in presence of traces of O2 or peroxide as initiator.

The mechanism of this reaction involves free radical addition and H-atom abstraction. The latter results in branching. As a result the chains are loosely held and the polymer has low density.
Properties of LDP :
- LDP films are extremely flexible, but tough chemically inert and moisture resistant.
- Itis poor conductor of electricity with melting point 110 °C.
Uses of LDP :
- LDP is mainly used in preparation of pipes for agriculture, irrigation, domestic water line connections as well as insulation to electric cables.
- It is also used in submarine cable insulation.
- It is used in producing extruded films, sheets, mainly for packaging and household uses like in preparation of squeeze bottles, attractive containers, etc.
(b) High density polyethylene( HDP) : It is a linear polymer with high density due to close packing.
![]()
HDP is obtained by polymerization of ethene in presence of Zieglar-Natta catalyst which is a combination of triethyl aluminium with titanium tetrachloride at a temperature of 333K to 343K and a pressure of 6-7 atm.
Properties of HDP :
- HDP is crystalline, melting point in the range of 144—150 °C.
- It is much stiffer than LDP and has high tensile strength and hardness.
- It is more resistant to chemicals than LDP.
Uses of HDP :
- HDP is used in manufacture of toys and other household articles like buckets, dustbins, bottles, pipes, etc.
- Ttis used to prepare laboratory wares and other objects where high tensile strength and stiffness is required.
(ii) Is synthetic rubber better than natural rubber ? If so, in what respect?
Yes.
- Synthetic rubber is superior to natural rubber in abrasion, heat resistance, and aging effects.
- It is flame-resistant, suitable for electrical insulation, and remains flexible at low temperatures.
- It is also resistant to grease, oil, heat, light, and certain chemicals.
- It is often used in insulation for electrical devices.
(iii) Write main specialties of Buna-S, Neoprene rubber?
- Buna-S is an elastomer and it is copolymer of styrene with butadiene. Its trade name is SBR. Buna-S is superior to natural rubber, because of its mechanical strength and abrasion resistance. It is used in tyre industry. It is vulcanized with sulphur.
- Neoprene is a synthetic rubber and it is a condensation polymer of chloroprene (2-chloro-1,3-butadiene). Vulcanization of neoprene takes place in presence of MgO. It is resistant to petroleum, vegetable oils. Neoprene is used in making hose pipes for transport of gasoline and making gaskets.
(iv) Write the structure of isoprene and the polymer obtained from it.
(v) Explain in detail free radical mechanism involved during preparation of addition polymer.
Free radical mechanism : Free radical mechanism is most common in addition polymerisation. It is also called chain reaction which involves three distinct steps chain initiation, chain propagation and chain termination.
Step 1 : Chain initiation : The chain reaction is initiated by a free radical.
An initiator (catalyst) such as benzoyl peroxide, acetyl peroxide, tert-butyl peroxide, etc. can be used to produce free radical. For example acetyl peroxide generates methyl radical as shown below :
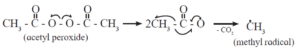
The free radical (say R) so formed attaches itself to the olefin (vinyl monomer) and produces a new radical, made up of two parts, namely, the attached radical and the monomer unit.
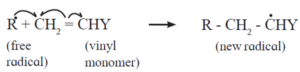
Step 2 : Chain propagation : The new radical formed in the initiation step reacts with another molecule of vinyl monomer, forming another still bigger sized radical, which in turn reacts with another monomer molecule. The repetition of this sequence takes place very rapidly. It is called chain propagation.
![]()
This step is very rapid and leads to high molecular mass radical.
Step 3 : Chain termination : Ultimately, at some stage, termination of the growing chain takes place. It may occur by several processes. One mode of termination is by combination of two growing chain radicals.
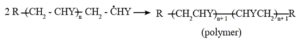
PDF : Class-12-Chemistry-Chapter-15-Introduction to Polymer Chemistry-Text Book
PDF : Class-12-Chemistry-Chapter-15-Introduction to Polymer Chemistry- Notes
PDF : Class-12-Chemistry-Chapter-15-Introduction to Polymer Chemistry- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 14- Biomolecules – Online Solutions
Next Chapter : Chapter-16-Green Chemistry and Nano chemistry- Online Solutions
We reply to valid query.