Alcohols, Phenols and Ethers
Maharashtra Board-Class-12-Chemistry-Chapter-11
Solutions
Question 1. Choose the correct option.
(i) Which of the following represents the increasing order of boiling points of (1), (2) and (3)?
(1) CH3-CH2-CH2-CH2-OH
(2) (CH3)2CHOCH3
(3) (CH3)3COH
(A) (1) < (2) < (3)
(B) (2) < (1) < (3)
(C) (3) < (2) < (1)
(D) (2) < (3) < (1)
(D) (2) < (3) < (1)
Explanation:
The boiling point of a compound is determined by its intermolecular forces. The stronger the intermolecular forces, the higher the boiling point. In this case, we can compare the compounds based on the strength of their intermolecular forces.
(1) CH3 - CH2 - CH2 - CH2 - OH is an alcohol, with the hydroxyl group (OH) capable of forming hydrogen bonds.
(2) (CH3)2CHOCH3 is a ketone, which can form dipole-dipole interactions.
(3) (CH3)3COH is a tertiary alcohol, also capable of forming hydrogen bonds.
Since hydrogen bonding is stronger than dipole-dipole interactions and tertiary alcohols have more steric hindrance, we can conclude that the increasing order of boiling points is (2) < (3) < (1).
(ii) Which is the best reagent for carrying out following conversion ?
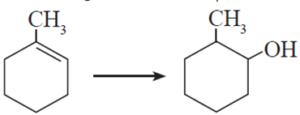
(A) LiAlH4
(B) Conc. H2SO4, H2O
(C) H2/Pd
(D) B2H6, H2O2-NaOH
(D) B2H6, H2O2-NaOH
Explanation:
This reaction is known as hydroboration-oxidation. Hydroboration-oxidation converts an alkene into alcohol with anti-Markovnikov regioselectivity, adding the hydroxyl group to the less substituted carbon atom.
(iii) Which of the following substrate will give ionic organic product on reaction ?
(A) CH3-CH2-OH + Na
(B) CH3-CH2-OH + SOCl2
(C) CH3-CH2-OH + PCl5
(D) CH3-CH2-OH + H2SO4
(A) CH3-CH2-OH + Na
Explanation:
When sodium combines with alcohol, it produces sodium alkoxide, which is a salt, and results in the release of hydrogen gas.
(iv) Which is the most resistant alcohol towards oxidation reaction among the
follwoing ?
(A) CH3-CH2-OH
(B) (CH3)2CH-OH
(C) (CH3)3C-OH
(D)
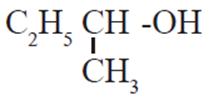
(C) (CH3)3C-OH
(v) Resorcinol on distillation with zinc dust gives
(A) Cyclohexane
(B) Benzene
(C) Toluene
(D) Benzene-1, 3-diol
(B) Benzene
(vi) Anisole on heating with concerntrated HI gives
(A) Iodobenzene
(B) Phenol + Methanol
(C) Phenol + Iodomethane
(D) Iodobenzene + methanol
(C) Phenol + Iodomethane
(vii) Which of the following is the least acidic compound ?
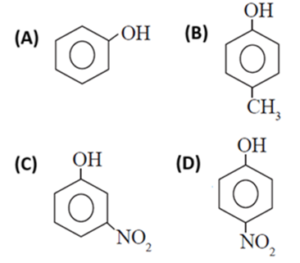
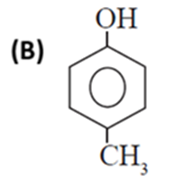
(viii) The compound incapable of hydrogen bonding with water is ......
(A) CH3-CH2-O-CH3
(B) CH3-CH2-CH2-CH3
(C)
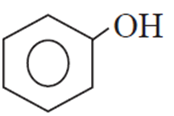
(D) CH3-CH2-CH2-OH
(B) CH3-CH2-CH2-CH3
(ix) Ethers are kept in air tight brown bottles because
(A) Ethers absorb moisture
(B) Ethers evaporate readily
(C) Ethers oxidise to explosive peroxide
(D) Ethers are inert
(C) Ethers oxidise to explosive peroxide
(x) Ethers reacts with cold and concentrated H2SO4 to form
(A) oxonium salt
(B) alkene
(C) alkoxides
(D) alcohols
(A) oxonium salt
Question 2. Answer in one sentence/ word.
(i) Hydroboration-oxidation of propene gives.....
n-propyl alcohol (CH3-CH2-CH2-OH)
(ii) Write the IUPAC name of alcohol having molecular formula C4H10O which is resistant towards oxidation.
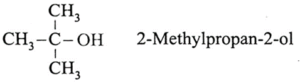
(iii) Write structure of optically active alcohol having molecular formula C4H10O
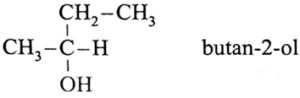
(iv) Write name of the electrophile used in Kolbe’s Reaction.
Electrophile : Carbon dioxide (O = C = O)
Question 3. Answer in brief.
(i) Explain why phenol is more acidic than ethyl alcohol.
The difference in the acidic character of phenols and alcohol is due to the difference in reactivity of these compounds towards the ionization of the O-H bond. This can be explained as follows:
- In ethyl alcohol, the -OH group is attached to sp3-hybridised carbon while in phenols, it is attached to sp2-hybridised carbon.
- Due to higher electronegativity of sp2-hybridised carbon, electron density on oxygen decreases. This increases the polarity of O-H bond and results in more ionization of phenol than that of alcohols.
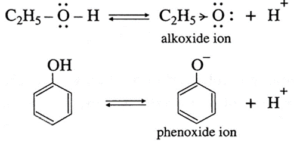
- Electron donating inductive effect (+ I effect) of the alkyl group destabilizes alkoxide ion. As a result alcohol does not ionize much in water, therefore alcohol is neutral compound in aqueous medium.
- In alkoxide ion, the negative charge is localized on oxygen, while in phenoxide ion the negative charge is delocalized. The delocalization of the negative charge (structure I to V) makes phenoxide ion more stable than that of phenol.
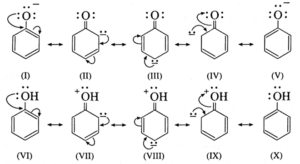
- The delocalization of charge in phenol (structures VI to X), the resonating structures have charge separation (where oxygen atom of OH group to be positive and delocalization of negative charge over the ortho and para positions of aromatic ring) due to which phenol molecule is less stable than phenoxide ion. This favours ionization of phenol. Thus phenols are more acidic than ethyl alcohol.
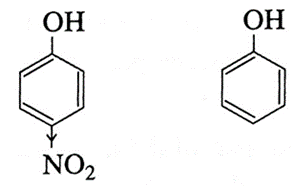
(ii) Explain why p-nitrophenol is a stronger acid than phenol.
- In p-nitrophenol, nitro group (NO2) is an electron withdrawing group present at para position which enhances the acidic strength (-I effect). The O-H bond is under strain and release of proton (H+) becomes easy. Further p-nitrophenoxide ion is more stabilised due to resonance.
- Since the absence of electron withdrawing group (like -NO2) in phenol at ortho and para position, the acidic strength of phenol is less than that of p-nitrophenol.
(iii) Write two points of difference between properties of phenol and ethyl alcohol.
| Phenol | Ethyl alcohol. |
| Phenol is a low melting solid. | Ethyl alcohol is liquid. |
| The aqueous solution of phenol turns blue litmus to red, i.e., phenol is weakly acidic. | The aqueous solution of ethyl alcohol is neutral to litmus, i.e, ethyl alcohol is neutral. |
| Phenol reacts with aqueous NaOH to form sodium phenoxide. | Ethyl alcohol does not react with aqueous NaOH. |
| Phenol reacts with neutral ferric chloride solution to give deep purple colouration of ferric phenoxide. | Ethyl alcohol does not react with neutral ferric chloride. |
(iv) Give the reagents and conditions necessary to prepare phenol from
(a) Chlorobenzene
(b) Benzene sulfonic acid.
(a) From chlorobenzene : Reagents required : NaOH and dil. HCl Temperature : 623 K, Pressure : 150 atm
(b) From Benzene sulphonic acid : Reagents required : aq. NaOH, caustic soda, dil. HCl Temperature : 573 K
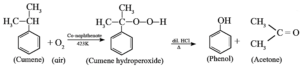
(v) Give the equations of the reactions for the preparation of phenol from isopropyl benezene.
Preparation of phenol from Cumene (Isopropyl benezene) :
- This is the commercial method of preparation of phenol.
- When a stream of air is passed through cumene (isopropylbenzene) suspended in aqueous Na2CO3 solution in the presence of cobalt naphthenate catalyst, isopropylbenzene hydroperoxide or cumene hydroperoxide is formed.
- Isopropylbenzene hydroperoxide on warming with dil. HCl gives phenol and acetone.
- Acetone is an important by-product of the reaction and is separated by distillation.
- The reaction is called auto oxidation.
(vi) Give a simple chemical test to distinguish between ethanol and ethyl bromide.
When ethyl bromide is heated with aq. NaOH; ethyl alcohol is formed whereas ethanol does not react with aq. NaOH
C2H3Br + aq. NaOH \(\underrightarrow{Δ}\) C2H5OH + NaBr
C2H5OH + aq. NaOH → No reaction
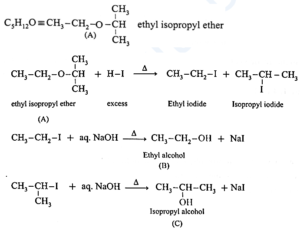
Question 4. An ether (A), C5H12O, when heated with excess of hot HI produce two alkyl halides which on hydrolysis form compound (B) and (C), oxidation of (B) gave and acid (D), whereas oxidation of (C) gave a ketone (E). Deduce the structural formula of (A), (B), (C), (D) and (E).
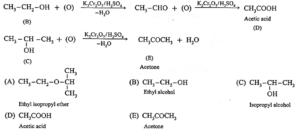
Question 5. Write structural formulae for
(a) 3-Methoxyhexane
(b) Methyl vinyl ether
(c) 1-Ethylcyclohexanol
(d) Pentane-1,4-diol
(e) Cyclohex-2-en-1-ol
(a) 3-Methoxyhexane :
![]()
(b) Methyl vinyl ether :
CH2=CH-OCH3
(c) 1-Ethylcyclohexanol :
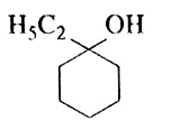
(d) Pentane-1,4-diol :
![]()
(e) Cyclohex-2-en-1-ol :
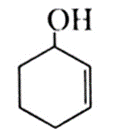
Question 6. Write IUPAC names of the following
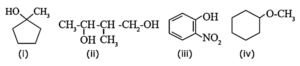
(i) 1-Methyl cyclopentanol
(ii) 2-Methyl butane-1, 3-diol
(iii) 2-Nitrophenol (o-nitrophenol)
(iv) Methoxy cyclohexane
PDF : Chapter-11-Alcohols, Phenols and Ethers-Text Book
PDF : Chapter-11-Alcohols, Phenols and Ethers- Notes
PDF : Chapter-11-Alcohols, Phenols and Ethers- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 10- Halogen Derivatives – Online Solutions
Next Chapter : Chapter-12-Aldehydes, Ketones and Carboxylic acids– Online Solutions
We reply to valid query.