Coordination Compounds
Maharashtra Board-Class-12-Chemistry-Chapter-9
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
Coordination compound : A coordination compound consists of central metal atom or ion surrounded by atoms or molecules.
Examples :
- Chemotherapy drug, cisplatin, Pt(NH3)2Cl2, is a coordination compound in which the central platinum metal ion is surrounded by two ammonia molecules and two chloride ions.
- [Cu(NH3)4]SO4
Ligand : The species surrounding the central metal atom or ion are called ligands.
Double salts : Double salts are crystalline molecular or addition compounds containing more than one salt in simple molecular proportions soluble in water and in solution they ionise and exhibit all the properties of the constituent ions.
Examples : K2SO4.A12(SO4)3.24H2O
K2SO4.Al2(SO4)3.24H2O(aq) → 2K+(aq) + 2Al3+(aq) + 4SO42-(aq) + 24H2O(l)
Lewis bases : In a coordination compound the ligands being electron pair donors they are Lewis bases.
- Example, in the coordination compound, [Cu(NH3)4]2+, NH3 is a Lewis base.
Lewis acids : The central metal atom or ion being electron acceptor behaves as a Lewis acid.
- Example, in the coordination compound, Cu2+ is a Lewis acid.
Types of ligands :
Ligands : The neutral molecules or negatively charged anions (or rarely positive ions) which are bonded by coordinate bonds to the central metal atom or metal ion in a coordination compound are called ligands or donor groups.
- Example : In [Cu(CN)4]2—, four CN— ions are ligands coordinated to central metal ion Cu2+.
- Ligands can be classified on the basis of number of electron donor atoms in the ligand i.e. denticity.
(1) Monodentate or unidentate ligand : A ligand molecule or an ion which has only one donor atom with a lone pair of electrons forming only one coordinate bond with metal atom or ion in the complex is called monodentate or unidentate ligand. For example NH3, Cl—, OH—, H2O, etc.
(2) Polydentate or multidentate ligand : A ligand molecule or an ion which has two or more donor atoms with the lone pairs of electrons forming two or more coordinate bonds with the central metal atom or ion in the complex is called polydentate or multidentate ligand. For example, ethylene diamine, H2N—(CH2)2—NH2. According to the number of donor atoms they are classified as follows :
- Bidentate ligand : This ligand has two donor atoms in the molecule or ion. For example, ethylenediamine, H2N—(CH2)2—NH2.
- Tridentate ligand : This ligand molecule has three donor atoms or three sites of attachment.E. g. Diethelene triamine, H2 — CH2-CH2— H—CH2-CH2— H2. This has three N donor atoms.
- Tetradentate (or quadridentate) ligand : This ligand molecule has four donor atoms. E. g. Triethylene tetraamine which has four N donor atoms.
- Hexdentate ligand : This ligand molecule has six donor atoms. E. g. Ethylenediamine tetracetato.
(3) Ambidentate ligand :A ligand molecule or an ion which has two or more donor atoms, however in the formation of a complex, only one donor atom is attached to the metal atom or an ion is called ambidentate ligand.
For example, NO2— which has two donor atoms N and O forming a coordinate bond, M ← ONO (nitrito) or M ← NO2 (nitro).
(4) Bridging ligand : A monodentate ligand having more than one lone pairs of electrons, hence can attach to two or more metal atoms or ions and hence acts as a bridge between different metal atoms is called bridging ligand.
For example : OH—, F—, SO4—2, etc.
Terms used in coordination chemistry :
The following terms are used for describing coordination compounds.
Coordination sphere : The central metal ion and ligands linked to it are enclosed in a square bracket. This is called a coordinationsphere, which is a discrete structural unit. The ionisable groups shown outside the bracket are the counter ions.
For example, the compound K4[Fe(CN)6] has [Fe(CN)6]4— coordination sphere with the ionisable K+ ions representing counter ions. The compound ionizes as :
K4[Fe(CN)6] → 4K+ + [Fe(CN)6]4—
Charge number of complex ion and oxidation state of metal ion :
Charge number of a complex ion : The net charge carried by a complex ion or a coordination entity is called its charge number.
- Charge number is equal to the algebraic sum of the charges carried by central metal atom or 1on and all the ligands attached to it.
- E.g. consider anionic complex, [Fe(CN)6]4—.
Charge number of [Fe(CN)6]4— = Charge on Fe2+ ions + 6 x charge on CN— = (+2) + 6(—1) = —4
Hence charge number of [Fe(CN)6]4— is —4.
Oxidation state of a metal in a complex : The oxidation state of a metal atom or ion in the complex is the apparent charge carried by it in the complex.
- It depends upon the atomic number and electronic configuration of the metal atom or ion.
- The coordination number, the formula and geometry of a complex depend upon the oxidation state of the metal atom or ion.
Q. What is the charge on a monodentate ligand X in the complex, [NiX4]2— ?
The charge number of the complex ion is — 2. Nickel being divalent, its oxidation state is + 2. If the charge on monodentate ligand X is y, then
Charge number = charge on Ni2+ + charge on 6X
— 2 = + 2 + 4 x y
∴ y = — 1
Hence the charge on ligand X is — 1.
Q. Calculate the oxidation state of a metal in the complexes, [Fe(NH3)6](NO3)3
[Fe(NH3)6](NO3)3 ⇌ [Fe(NH3)6]3+ + (NO3)3—
NH3 is a neutral ligand, and the charge number of complex ion is + 3.
If the oxidation state of Fe is x then,
+ 3 = x + 6(0)
∴ x = + 3
The oxidation state of Fe is + 3.
Coordination number (C.N.) of central metal ion :
Coordination number (C.N.) of metal ion in a complex is the number of ligand donor atoms directly attached to it or the number of electron pairs involved in the coordinate bond.
Explanation :
- The coordination number (C.N.) is a characteristic property of the metal and its electronic configuration
- C.N. takes the values from 2 to 10, of which 4 and 6 are very common.
- The light transition metals show C.N. 4 and 6 while the heavier transition metals show C.N.
- The geometry and shape of a complex compound depends upon C.N. of the metal.
Coordination number of Co in [CoCl2(en)2]+ = 6
Coordination number of Ir in [Ir(C2O4)2Cl2]3+ = 6
Coordination number of Pt in [Pt(NO2)2(NH3)2] = 6
| Know This :
(i) Coordination number used in coordination of compounds in somewhat different than that used in solid state. Explanation :
|
Double salt and coordination complex :
Combination of two or more stable compounds in stochiometric ratio can give two types of substances, namely, double salt and coordination complexes.
Double salt : A double salt dissociates in water completely into simple ions.
- Double salts lose their identity in the solution.
- The properties of double salts are same as those of their constituents.
- Metal ions in the double salts show their normal valence.
Examples :
(i) Mohr's salt, FeSO4(NH4)2SO4.6H2O dissociates as :
FeSO4(NH4)2SO4.6H2O Fe2+(aq) + 2NH4+(aq) +2SO42— (aq)
(ii) Carnalite KCl.MgCl2.6H2O dissociates as
KCl.MgCl2.6H2O K+ (aq) + Mg2+ (aq) + 3Cl—(aq)
Coordination complex : A coordination complex dissociates in water with at least one complex ion.
- They do not lose their identity completely.
- The properties of coordination compounds are different from their constituents.
- Metal ions in the coordination compounds show two valences namely primary valence and secondary Valence satisfied by anions or neutral molecules called ligands.
Example : K4[Fe(CN)6] dissociates as the complex ion and counter ion.
K4[Fe(CN)6] → 4K+(aq) + [Fe(CN)6]4—
(counter ion) (complex ion)
Remember : When a complex is brought into solution it does not dissociate into simple metal ions. When [Co(NH3)6]Cl3 is dissolved in water it does not give the test for Co3+ or NH3. However, on reacting with AgNO3 a curdy white precipitate of AgCl corresponding to 3 moles is observed.
Werner theory of coordination complexes :
The first attempt to explain nature of bonding in coordination compounds was put forth by Werner. The postulates of Werner theory are as follows.
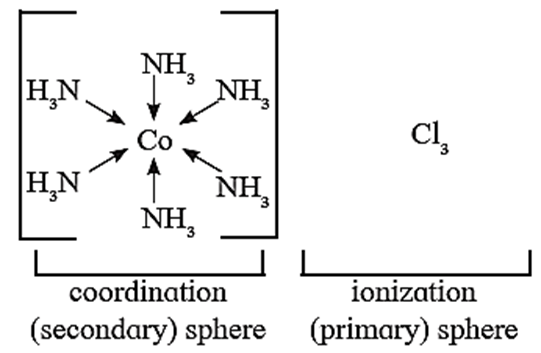
Postulate (i) : Unlike metal salts, the metal in a complex possesses two types of valencies : primary (ionizable) valency and secondary (nonionizable) valency.
Postulate (ii) : The ionizable sphere consists of entities which satisfy the primary valency of the metal. Primary valencies are generally satisfied by anions.
Postulate (iii) : The secondary coordination sphere consists of entities which satisfy the secondary valencies and are non ionizable.
The secondary valencies for a metal ion are fixed and satisfied by either anions or neutral ligands. Number of secondary valencies is equal to the coordination number.
Postulate (iv) : The secondary valencies have a fixed spatial arrangement around the metal ion. Two spheres of attraction in the complex [Co(NH3)6]Cl3 are shown.
Classification of complexes:
The coordination complexes are classified according to types of ligands and sign of charge on the complex ion.
Classification on the basis of types of ligands :
- (i) Homoleptic complexes : Consider [Co(NH3)6]3+. Here only one type of ligands surrounds the Co3+ ion. The complexes in which metal ion is bound to only one type of ligands are homoleptic.
- (ii) Heteroleptic complexes : Look at the complex [Co(NH3)4Cl2]+. There are two types of ligands, NH3 and Cl attached to Co3+ ion. Such complexes in which metal ion is surrounded by more than one type of ligands are heteroleptic.
Examples :
- Homoleptic Complexes : [Cu(C2O4)3]3—
- Heteroleptic Complexes : [Co(NH3)5Cl]SO4, [Co(ONO)(NH3)5]Cl2, [CoCl(NH3)(en)2]2+
Classification on the basis of charge on the complex :
(i) Cationic complexes : A positively charged coordination sphere or a coordination compound having a positively charged coordination sphere is called cationic sphere complex.
For example: the cation [Zn(NH3)4]2+ and [Co(NH3)5Cl]SO4 are cationic complexes. The latter has coordination sphere [Co(NH3)5Cl] 2+; the anion SO42— makes it electrically neutral.
(ii) Anionic sphere complexes : A negatively charged coordination sphere or a coordination compound having negatively charged coordination sphere is called anionic sphere complex.
For example, [Ni(CN)4]2— and K3[Fe(CN)6] have anionic coordination sphere; [Fe(CN)6]3— and three K+ ions make the latter electrically neutral.
(iii) Neutral sphere complexes : A neutral coordination complex does not possess cationic or anionic sphere. [Pt(NH3)2Cl2] or [Ni(CO)4] have neither cation nor anion but are neutral sphere complexes.
IUPAC nomenclature of coordination compounds :
Rules for naming coordination compounds recommended by IUPAC are as follows:
(i) In naming the complex ion or neutral molecule, name the ligand first and then the metal.
(ii) The names of anionic ligands are obtained by changing the ending -ide to -o and -ate to -ato.
(iii) The name of a complex is one single word. There must not be any space between different ligand names as well as between ligand name and the name of the metal.
(iv) After the name of the metal, write its oxidation state in Roman number which appears in parentheses without any space between metal name and parentheses.
(v) If complex has more than one ligand of the same type, the number is indicated with prefixes, di-, tri-, tetra-, penta-, hexa- and so on.
(vi) For the complex having more than one type of ligands, they are written in an alphabetical order. Suppose two ligands with prefixes are tetraaqua and dichloro. While naming in alphabetical order, tetraaqua is first and then dichloro.
(vii) If the name of ligand itself contains numerical prefix then display number by prefixes with bis for 2, tris for 3, tetrakis for 4 and so forth. Put the ligand name in parentheses. For example, (ethylenediamine)3 or (en3) would appear as tris(ethylenediamine) or tris(ethane-1, 2-diamine).
(viii) The metal in cationic or neutral complex is specified by its usual name while in the anionic complex the name of metal ends with 'ate'.
Below tables summarize the IUPAC nomenclature of coordination compounds :
Table - IUPAC names of anionic and neutral ligands :
| Anionic ligand | IUPAC name | Anionic ligand | IUPAC name |
| Br—, Bromide | Bromo | CO32—, Carbonate | Carbonato |
| Cl—, Chloride | Chloro | OH—, Hydroxide | Hydroxo |
| F—, Fluoride | Fluoro | C2O42—, Oxalate | Oxalato |
| I—, Iodide | Iodo | NO2—, Nitrite | Nitro (For N - bonded ligand) |
| CN—, Cyanide | Cyano | ONO—, Nitrite | Nitrito(For O-bonded ligand) |
| SO42—, sulphate | Sulphato | SCN—, Thiocyanate | Thiocyanato (For ligand do- nor atom S) |
| NO3 , Nitro | Nitrato | NCS—, Thiocyanate | Isothiocyanato (For ligand donor atom N) |
| Neutral ligand | IUPAC name | Neutral ligand | IUPAC names |
| NH3, Ammonia | Ammine (Note the spelling) | H2O, water | Aqua |
| CO, Carbon monoxide | Cabonyl | en, Ethylene diamine | Ethylenediamine |
Table - IUPAC names of metals in anionic complexes :
| Metal | IUPAC name | Metal | IUPAC name |
| Aluminium, Al | Aluminate | Chromium, Cr | Chromate |
| Cobalt, Co | Cobaltate | Copper, Cu | Cuprate |
| Gold, Au | Aurate | Iron, Fe | Ferrate |
| Manganese, Mn | Maganate | Nickel, Ni | Nickelate |
| Platinum, Pt | Platinate | Zinc, Zn | Zincate |
Table - IUPAC names of some complexes :
| Complex | IUPAC name |
| i. Anionic complexes :
a.[Ni(CN)4]2— b. [Co(C2O4)3]3— c. [Fe(CN)6]4— |
Tetracyanonickelate(II) ion Trioxalatocobaltate(III) ion Hexacyanoferrate(II) ion |
| ii. Compounds containing complex anions and metal cations : | |
| a. Na3[Co(NO2)6]
b. K3[Al(C2O4)3] c. Na3[AlF6] |
Sodium hexanitrocobaltate(III)
Potasium trioxalatoaluminate(III) Sodium hexafluoroaluminate(III) |
| iii. Cationic complexes :
a. Cu(NH3)42+ b. [Fe(H2O)5(NCS)] 2+ c. [Pt(en)2(SCN)2] 2+ |
Tetraamminecopper(II) ion Pentaaquaisothiocyanatoiron(III) ion, Bis(ethylenediamine)dithiocyanatoplatinum(IV). |
| iv. Compounds containing complex cations and anion : | |
| a.[PtBr2(NH3)4]Br2
b. [Co(NH3)5CO3]Cl c. [Co(H2O)(NH3)5]I3 |
Tetraamminedibromoplatinum(IV) bromide,
Pentaamminecarbonatocobalt(III) chloride, Pentaammineaquacobalt(III) iodide. |
| v. Neutral complexes :
a. [Co(NO2)3(NH3)3] b. Fe(CO)5 c. [Rh(NH3)3(SCN)3] |
Triamminetrinitrocobalt(III) Pentacarbonyliron(0) Triamminetrithiocyanatorhodium(III) |
Effective Atomic Number (EAN) Rule :
Effective atomic number (EAN) : It is the total number of electrons present around the central metal atom or ion and calculated as the sum of electrons of metal atom or ion and the number of electrons donated by ligands.
EAN is calculated by the formula : EAN = Z — X + Y
where, Z = Atomic number of metal atom
X = Number of electrons lost by a metal atom forming a metal ion
Y = Total number of electrons donated by all ligands in the complex.
Generally the value of EAN is equal to the atomic number of the nearest inert element.
Explanation :
Consider a complex ion [Co(NH3)6]3+
Oxidation state of cobalt is + 3 hence X = 3.
There are six ligands, hence Y = 2 x 6 = 12
Atomic number of cobalt, Z = 27
∴ EAN = Z — X + Y = 27 — 3 + 12 = 36.
EAN rule : It states that a metal ion continues to accept electrons pairs till it attains the electronic configuration of the next noble gas. Thus if the EAN is equal to 18 (Ar), 36 (Kr), 54 (Xe), or 86 (Rn) then the EAN rule is obeyed.
Cr(CO)6 and [Fe(CN)6]4 are some examples of coordination compounds which obey the EAN rule. Certain other coordination compounds however do not obey the EAN rule.
Example :
(a) Cr(CO)4 : EAN = Z — X + Y = 24 – 0 + 8 =32
(b) Mn(CO)5 : EAN = Z — X + Y = 25 – 0 + 10 =35
∴ Cr(CO)4 and Mn(CO)5 do not follow EAN Rule
Similarly [Fe(CN)6]3 and Cu[NH3]42+ do not obey the EAN rule have EAN 35.
Isomerism in coordination compounds :
Isomerism : It is the phenomenon in coordination compounds having same molecular formula but different physical and chemical properties due to different arrangements of the ligands around the central metal atom or ion in the space.
Isomers : The isomers are the coordination compounds having same molecular formula but different physical and chemical properties due to the difference in arrangements of the ligands in the space.
- Isomers are classified into two types namely stereoisomers and constitutional (or structural) isomers
- Stereoisomerism is further classified as : (i) Geometrical isomerism (ii) Optical isomerism.
- Structural isomerism is further classified as (i) Ionisation isomerism (ii) Linkage isomerism (iii) Coordination isomerism (iv) Solvate (or hydrate) isomerism.
(1) Stereoisomers : Stereoisomers have the same links among constituent atoms however the arrangements of atoms in space are different.
- In the coordination compounds (complexes) the ligands are linked to the central metal atom or ion by coordinate bonds which are directional in nature and hence give rise to the phenomenon of stereoisomerism.
- In this isomerism, the different stereoisomers have different arrangements of ligands (atoms, molecules or ions) in space around the central metal atom or ion. Hence they have different physical and chemical properties and give rise to the phenomenon of stereoisomerism.
Stereoisomerism : The phenomenon of isomerism in the coordination compounds arising due to different spatial positions of the ligands in the space around the central metal atom or ion is called stereoisomerism.
Stereoisomers : The coordination compounds having same molecular formula but different stereoisomerism due to different spatial arrangements of the ligand groups in the space around the central metal atom or ion are called stereoisomers.
Geometrical isomerism : The phenomenon of isomerism in the heteroleptic coordination compounds with the same molecular fonnula but different spatial arrangement of the ligands in the space around the central metal atom or ion is called geometrical isomerism.
Geometrical isomers : The heteroleptic coordination compounds having same molecular formula but different geometrical isomerism due to different spatial arrangements of the ligands in the space around the central metal atom or ion are called geometrical isomers.
These are nonsuperimposable mirror image isomers. These are possible in heteroleptic complexes. In these isomers, there are cis and trans types of arrangements of ligands.
- Cis-isomers : Identical ligands occupy adjacent positions.
- Trans-isomer : Identical ligands occupy the opposite positions.
Cis and trans isomers have different properties. Cis trans isomerism is observed in square planar and octahedral complexes.
The square planar complexes of MA2B2 and MA2BC type exist as cis and trans isomers, where A, B and C are monodentate ligands, M is metal.
For example : Pt(NH3)2Cl2, (MA2B2 type)
(i) Cis-isomer : A heteroleptic coordination compound in which two similar ligands are arranged adjacent to each other is called cis-isomer.
For example, Cis-Diamminedichloroplatinum(II)
(ii) Trans-isomer : A heteroleptic coordination compound in which two similar ligands are arranged diagonally opposite to each other is called trans-isomer.
For example, Trans-Diamminedichloroplatinum (II)
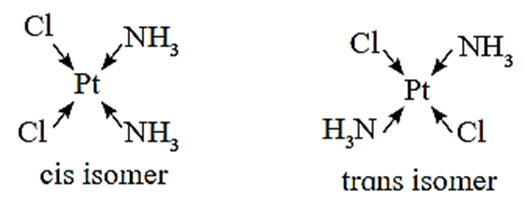
- Here the cis isomer is more soluble in water than the trans isomer. The cis isomer named cisplatin is an anticancer drug while the trans isomer is physiologically inactive.
- The cis isomer is polar with non-zero dipole moment. The trans isomer has zero dipole moment as a result of the two opposite Pt – Cl and two Pt-NH3 bond moments, which cancel each other.
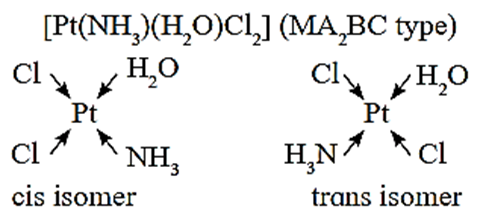
- Four coordinate tetrahedral complexes do not show cis and trans isomers.
Cis and trans isomers in octahedral complexes :
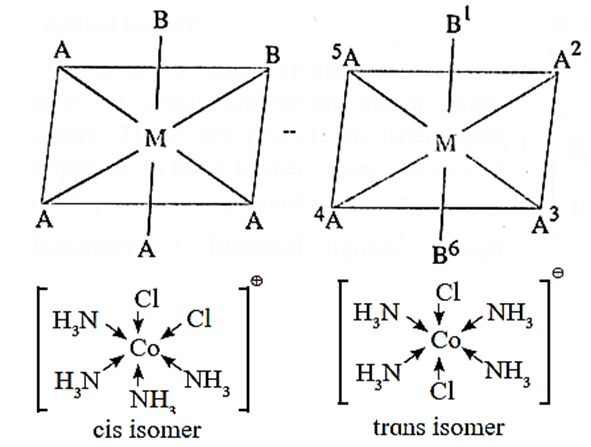
Geometrical isomerism of the octahedral complex of the type [MA4B2] :
Consider an octahedral complex of a metal M with coordination number six and monodentate ligands a and b having formula [MA4B2].
- Cis-isomer is obtained when both the B ligands occupy adjacent (1, 2) positions.
- Trans-isomer is obtained when the ligands B occupy the opposite (1, 6) positions.
- For example, consider a complex [Co(NH3)4Cl2]+. The structures of cis and trans isomers are :
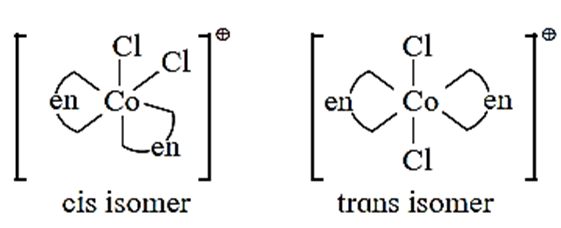
Geometrical isomerism of the octahedral complex of the type [M(AA)2B2]n± :
- Consider an octahedral complex of metal M with coordination number six and a bidentate ligand AA and monodentate ligand B having molecular formula [M(AA)2B2]n±
- Bidentate ligand AA has two identical coordinating atoms.
- Cis - isomer is obtained when two bidentate AA ligands as well as two ‘B’ ligands are at adjacent positions.
- Trans-isomer is obtained when two AA ligands and two B ligands are at opposite positions.
- For example, consider a complex [Co(en)2Cl2]+
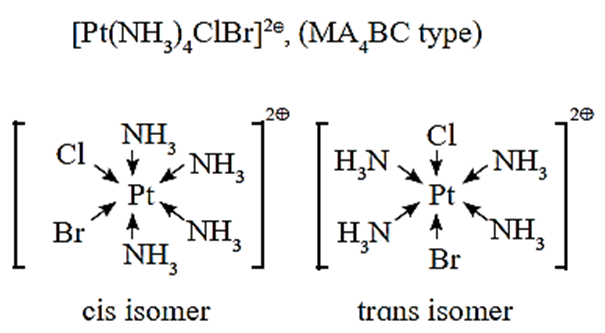
Geometrical isomerism of the octahedral complex of the type [MA4BC] :
- Consider an octahedral complex of metal M with coordination number six and monodentate ligands A, B and C.
- Cis-isomer is obtained when both the ligands B and C occupy adjacent (1, 2) positions.
- Trans-isomer is obtained when the ligands B and C occupy opposite positions.
- For example, consider a complex [Pt(NH3)4BrCl] of the type [MA4BC].
Optical isomerism : The phenomenon of isomerism in which different coordination compounds having same molecular formula have different optical activity is called optical isomerism.
Optical isomers : Different coordination compounds having same molecular formula but different optical activity are called optical isomers.
Plane polarised light : A monochromatic light having vibrations only in one plane is called a plane polarized light. This light is obtained by passing monochromatic light through NICOL prism.
Optical activity : A phenomenon of rotating a plane of a plane polarised light by an optically active substance is called optical activity. This substance is said to be optically active.
Dextrorotatory substance : An optically active substance which rotates the plane of a plane polarised light to right hand side is called dextrorotatory or d isomer denoted by d.
Laevorotatory substance : An optically active substance which rotates the plane of a plane polarised light to the left hand side is called laevorotatory or Z isomer and denoted by l
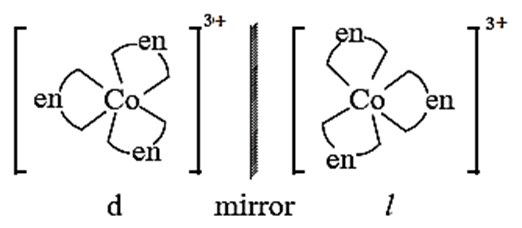
Enantiomers : The two forms of the optical active complex molecule which are mirror images of each other are called enantiomers. There are two forms of enantiomers, d form and l form.
Chiral :
When the mirror images of optical isomers of the complex are nonsuperimposable they are said to be chiral. For example, [C0(en)2(NH3)2]3+.
(i) Optical iomers in octahedral complexes, [Co(en)3]3+ :
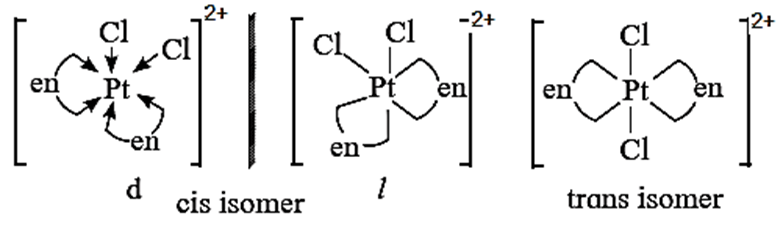
(ii) Octahedral complexes existing as both geometric and optical isomers [PtCl2(en)2]2+
- The octahedral complexes of the type [M(AA)2Q2]n±, in which two symmetrical bidentate chelating ligands like AA and two monodentate ligands like a are coordinated to the central metal atom or ion exhibit optical isomerism and two optical isomers d and l can be resolved. For example, [PtCl2(en)2]2+
- The cis-form is unsymmetrical and optically active while trans-form is symmetrical and hence optically inactive.
The optical isomers of cis-form (d and l ) of this complex along with trans-form are shown below,
Square planar complexes do not show enantiomers since they have mirror plane and axis of symmetry.
Remember : Our hands are nonsuperimposable mirror images. When you hold your left hand up to a mirror the image looks like right hand.
Structural isomers (Constitutional isomers) :
Structural isomers possess different linkages among their constituent atoms and have, their chemical formulae to be the same.
They can be classified as linkage isomers, ionization isomers, coordination isomers and solvate isomers.
(i) Linkage isomers :
Linkage isomerism : The phenomenon of isomerism in which the coordination compounds have same metal atom or ion and same ligand but bonded through different donor atoms or linkages is known as linkage isomerism.
Linkage isomers : The coordination compounds having same metal atom or ion and ligand but bonded through different donor atoms or linkages are called linkage isomers.
Example : Nitro complex [Co(NH3)5NO2]Cl2 (Yellow) and nitrito complex [Co(NH3)5ONO]Cl2 (Red)
- The nitrite ion NO2 having two donor atoms.
- The nitro complex has Co-N bond and the nitrito complex is linked through Co-O bond. These are linkage isomers.
(ii) Ionization isomers :
The coordination compounds having same molecular composition but differ in the compositions of coordination (or inner) sphere and outer sphere and produce different ions on ionisation in the solution are called ionisation isomers.
Examples :
- (i) Pentaamminesulphatocobalt (III) bromide [Co(NH3)5SO4] Br,
- (ii) Pentaamminebromocobalt(III) sulphate [Co(NH3)5Br] SO4.
In compound (i), anion SO42—, bonded to Co is in the coordination sphere while Br— is in the ionization sphere.
In compound (ii), anion Br— is in the coordination sphere linked to Co while SO42— is in the ionisation sphere.
(iii) Coordination isomers :
Coordination isomerism : The phenomenon of isomerism in the ionic coordination compounds having the same molecular formula but different complex ions involving the interchange of ligands between cationic and anionic spheres of different metal ions is called coordination isomerism.
Coordination isomers : The ionic coordination compounds having same molecular formula but different complex ions due to interchange of ligands between cationic and anionic spheres of different metal ions are called coordination isomers.
Examples:
(a) [Co(NH3)6]3+ [Cr(CN)6]3— and (ii) [Cr(NH3)6] 3+ [Co(CN)6]3—
(cationic) (anionic) (cationic) (anionic).
- In isomer (i) cobalt is linked to ammine ligand and chromium to cyanide ligand.
- In isomer (ii) the ligands coordinating to metals are interchanged. Cobalt coordinates with cyanide ligand and chromium to NH3 ligand.
(b) [Cu(NH3)4][PtCl4] and [Pt(NH3)4][CuCl4]
(c) [Cr(NH3)6][Cr(SCN)6] and [Cr(NH3)4(SCN)2][Cr(SCN)4(NH3)2]
(iv). Solvate isomers (Hydrate isomers when water is solvent) :
The coordination compounds having the same molecular formula but differ in the number of solvent or H2O molecules inside the coordination sphere and outer sphere of the complexes are called solvate or hydrate isomers.
Examples :
- (a)[Cr(H2O)6]Cl3; [Cr(H2O)5Cl]Cl2.H2O; and [Cr(H2O)4Cl2]Cl.2H2O.
- (b) The possible hydrate isomers of the coordination compounds having molecular formula CoCl3.6H2O are as : (i) [Co(H2O)6]Cl3, (ii) [Co(H2O)5Cl]Cl2.H2O, (iii) [Co(H2O)4Cl2]Cl.2H2O, (iv) [Co(H2O)3Cl3].3H2O.
PDF : Chapter-9-Coordination Compounds-Text Book
PDF : Chapter-9-Coordination Compounds- Notes
PDF : Chapter-9-Coordination Compounds- Solution
All 16 Chapters Notes -Class-12-Chemistry (16-PDF)
All 16 Chapters Solutions -Class-12-Chemistry (16-PDF)
All 16 Chapters Notes+Solutions -Class-12-Chemistry (32-PDF)
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 8- Transition and Inner transition Elements – Online Notes
Next Chapter : Chapter-10-Halogen Derivatives– Online Notes
We reply to valid query.