Aldehydes, Ketones and Carboxylic acids
Maharashtra Board-Class-12-Chemistry-Chapter-12
Notes-Part-1
|
Topics to be Learn : Part-1
|
Introduction :
Carbonyl group : A functional group in which a carbon atom is attached to an oxygen atom by a double bond and remaining two valencies of carbon atom are free is called a carbonyl group and represented as (>C=O ). Carbonyl group is present in aldehydes and ketones.
Carbonyl compounds : The organic compounds containing a carbonyl group (>C=O)are called carbonyl compounds.
For example, acetaldehyde, ![]() , acetone,
, acetone,![]()
Carbonyl compounds are classified as follows :
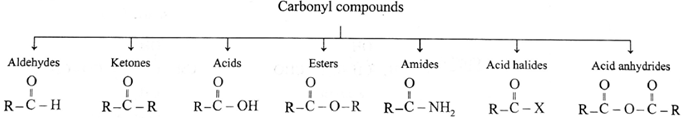
As carbonyl group is common in aldehydes and ketones, their methods of preparation and properties show similarities.
Carboxylic compounds : The compounds in which the functional group is —COOH are known as carboxylic compounds. Due to the —OH group bonded to (>C=O ) group, carboxylic acids are distinct from aldehydes and ketones.
Classification of aldehydes, ketones and carboxylic acids :
Aldehydes, ketones and carboxylic acids are classified as per the nature of carbon skeleton bonded to (>C=O ).
Classification of aldehydes :
Aldehydes are classified as aliphatic and aromatic aldehydes
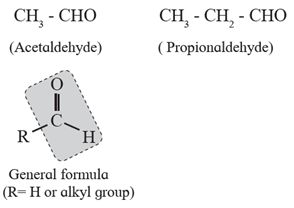
(i) Aliphatic aldehydes : The compounds in which the –CHO group (formyl group) is
attached directly to sp3 hybridized carbon atom that is saturated carbon atom are
called aliphatic aldehydes. (Exception : Formaldehyde, H-CHO is also classified as
aliphatic aldehyde though –CHO group is not attached to any carbon ).
For example :
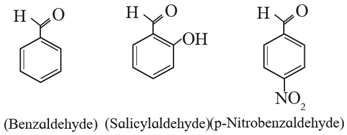
(ii) Aromatic aldehydes : The compounds in which –CHO group is attached directly to an aromatic ring are called aromatic aldehydes.
For example :
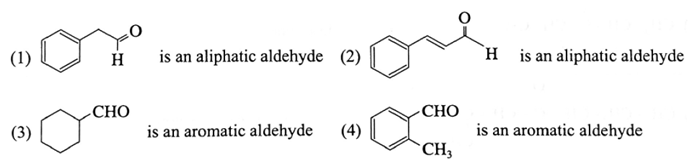
Examples of aliphatic and aromatic aldehydes :
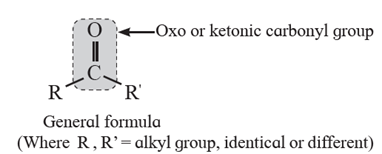
Structure of carbonyl functional group :
- In the carbonyl functional group, carbon atom is attached to an oxygen atom by a double bond and remaining two valencies of carbon atom are free, and it is represented as >C=0.
- The carbonyl carbon atom is sp2-hybridised forming coplanar three sigma (σ) bonds with the bond angle 120°.
- One sigma bond is formed with oxygen atom while other two sigma (σ) bonds are formed with hydrogen or carbon atoms.
- The remaining unhybridised 2pz orbital of carbon atom overlaps with p orbital of oxygen atom collaterally forming a pi (π) bond. Hence, carbon atom is joined to oxygen atom by a double bond of which one is sigma and another is π.
- The oxygen atom in the carbonyl group has two lone pairs of electrons.
- The carbonyl bond is strong, short and polarized.
- The polarity of the carbonyl group is explained on the basis of resonance involving a neutral and dipolar structures as shown below :
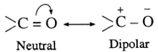
Classification of ketones : Ketones are classified as aliphatic and aromatic ketones:
(i) Aliphatic ketones : The compounds in which >C=O group is attached to two alkyl groups are called aliphatic ketones.
Examples :
Ketones are classified into two types :
- Simple or symmetrical ketones
- mixed or unsymmetrical
(a) Simple or symmetrical ketone : The ketone in which the carbonyl carbon is attached to two identical alkyl groups is called a simple or symmetrical ketone.
Examples :

(b) Mixed or unsymmetrical ketones : The ketones in which two alkyl groups bonded to carbonyl carbon are different, are called mixed ketones or unsymmetrical ketones.
Example :
(ii) Aromatic ketones : The compounds in which a >C=O group is attached to either two aryl groups or one aryl and one alkyl group are called aromatic ketones.
Example :
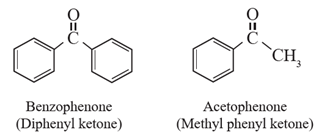
Question. Classify the followings as simple and mixed ketones.
- Benzophenone, acetone, butanone, acetophenone.
| Simple ketones | Mixed ketones |
| Benzophenone, acetone | butanone, acetophenone |
Know This :
|
Classification of carboxylic acids :
Carboxylic acids are classified as aliphatic and aromatic carboxylic acids :
(i) Aliphatic carboxylic acids : The organic compounds in which carboxyl (-COOH) group is bonded to an alkyl group are called aliphatic carboxylic acids or fatty acids. (Exception : Formic acid, H-COOH is also classified as aliphatic carboxylic acid though –COOH group is not attached to any carbon).
Example :
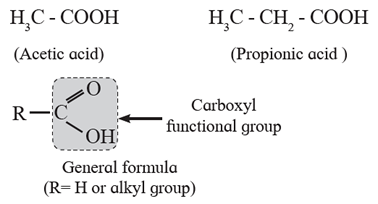
Depending on the number of –COOH groups present carboxylic acids are classified as mono, di, tri carboxylic acids and so on.
Examples :
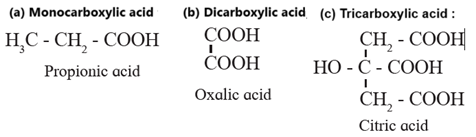
Monocarboxylic acid contain one carboxyl group, dicarboxylic acid contain two carboxyl group and Tricarboxylic acid contain three carboxyl group .
(ii) Aromatic carboxylic acids : These are the compounds in which one or more carboxyl groups (-COOH ) are attached directly to the aromatic ring.
Examples :
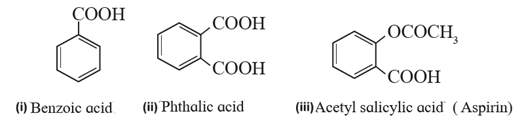
The aromatic compounds in which the –COOH group is not attached directly to the ring are called side-chain aromatic acids.
Example :

Examples of common carboxylic acids which are used in daily life :
Common carboxylic acids are widely distributed in nature, they are found in both the plants and animals.
- Acetic acid is main constituent of vinegar.
- Butyric acid of butter which is responsible for odour of rancid butter.
- L-lactic acid is present in curd.
- Citric acid is found in citrus fruits.
- Higher carboxylic acids such as palmitic acid, stearic acid and oleic acid are the components of animal fats and vegetable oils.
Nomenclature of aldehydes, ketones and carboxylic acids :
Nomenclature of aldehydes and carboxylic acid : The names of aldehydes and carboxylic acids are related to each other. There are two systems of naming aldehydes and carboxylic acids : trivial and IUPAC.
Nomenclature of Aldehydes :
(i) Common System :
- The names of aldehydes are derived from the common names of acids.
- The suffix ‘- ic acid’ of an acid is replaced by ‘aldehyde’.
- The positions of the substituents in the molecule are indicated by Greek letters α β, ν, etc. starting from the carbon atom attached to the carbonyl group.
Example :
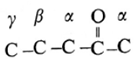
(ii) IUPAC System :
- The longest carbon atoms chain containing aldehyde carbon atom is selected as a parent hydrocarbon.
- ‘e’ of the alkane is replaced by ‘al’. Alkane → Alkanal
- The position (locant) of aldehyde group need not be mentioned since it is always at the end position.
- The substituents in the alkyl group are prefixed in an alphabetical order by appropriate locants.
- When two —CHO groups are present at the two ends of the chain the ending ‘e’ of alkane is retained and the suffix ‘—dial’ is added to the name of parent aldehyde.
- In IUPAC nomenclature an alicyclic compound in which —CHO group is attached directly to the ring is named as a carbaldehyde. The suffix ‘carbaldehyde’ is added after the full name of parent cycloalkane structure.
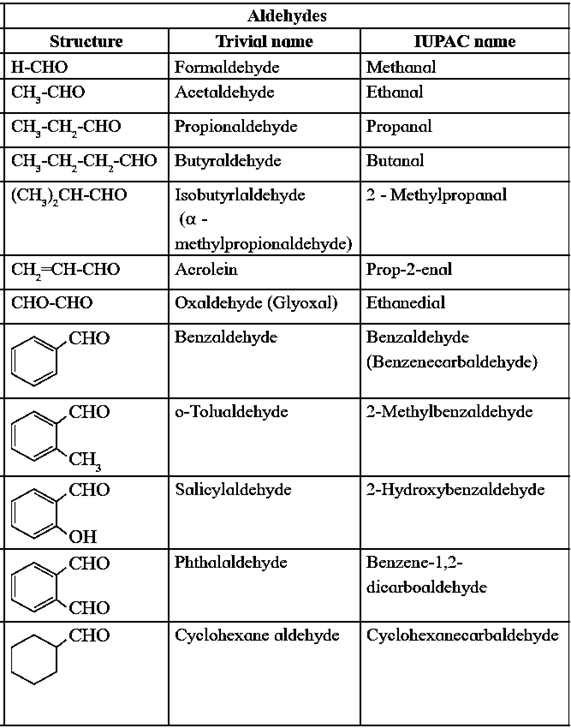
Nomenclature of carboxylic acid :
(i) Common or trivial names :
The trivial names of carboxylic acids are often derived from Latin names of their original natural source.
For example, Common names and the source or origin of name of Carboxylic acid as given below :
- Formic acid (H–COOH) is obtained from red ants (Formica means ant),
- Acetic acid (CH3–COOH) is obtained from acetum (acetum means vinegar),
- Propionic acid (CH3–CH2–COOH) is from basic fat (propion means first fat),
- Butyric acid (CH3–CH2 –CH2–COOH) is from butter (butyrum means butter).
- Valeric acid (CH3–CH2 –CH2– CH2–COOH) is from valerian (a perennial plant)
- Caproic acid (CH3–CH2–CH2– CH2– CH2–COOH) is from Caper (goat)
In branched carboxylic acids, the position of substituents are indicated by Greek alphabet.
Example :
![]()
(ii) IUPAC system of nomenclature :
- The longest continuous chain of carbon atoms including the carbon atom of — COOH group is selected. The carboxylic acid is considered as a derivative of the corresponding parent alkane.
- The name of carboxylic acid is obtained by replacing ‘e’ from the name of parent alkane by — ‘oic acid’. Thus carboxylic acids are called alkanoic acids.
- The carbon atom of the —COOH group is always at terminal position, hence need not to be indicated while writing IUPAC name.
- The position of the other substitutents are indicated by the appropriate locants in alphabetical order.
- In case of dicarboxylic acids, ‘dioic acid’ is added to parent alkane.
- In an alicyclic compound having a carboxyl group directly attached to alicyclic ring is named as cycloalkane carboxylic acid.
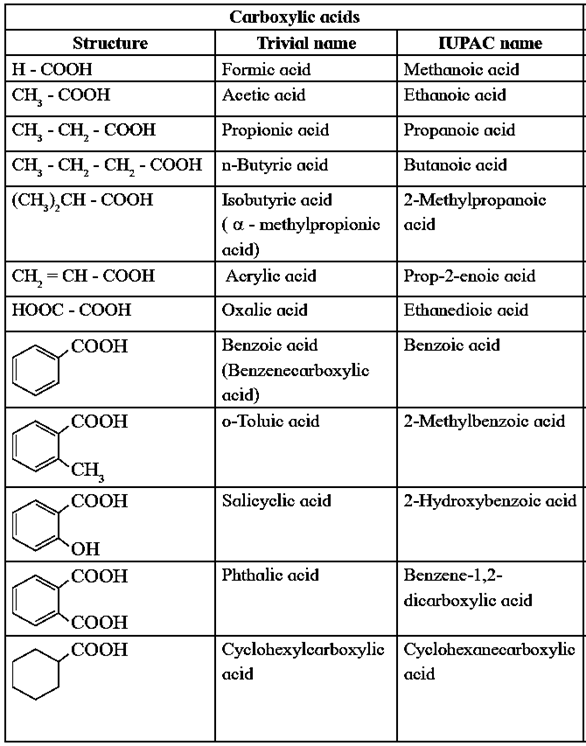
Substituted aromatic aldehydes and carboxylic acids : When two or more different functional groups are attached to a ring , the higher priority group is given lower number. When –CHO group, appears as substituent prefix ‘formyl’ is used in the IUPAC name.
Examples:
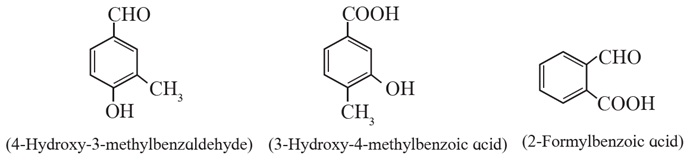
| Know This :
(i) Series of straight chain dicarboxylic acids are commercially known by the following common names :
(ii) A series of lower fatty acids are commercially known by the following common names.
|
Trivial and IUPAC names of ketones:
Nomenclature of ketones :
(i) The trivial names :
- Ketones are named according to the alkyl groups attached to the carbonyl carbon atom followed by the word ketone.
- The substituents in the alkyl groups are indicated by Greek letters α β, ν, etc. starting from the carbon atom attached to the carbonyl group.
(ii) IUPAC System :
- The longest continuous chain containing carbonyl carbon atom is selected as a parent hydrocarbon.
- ‘e’ of the alkane is replaced by ‘one’. Alkane — Alkanone
- The position of carbonyl group is represented by the lowest locant.
- The substituents in the alkyl groups are prefixed in the alphabetical order along with their positions by appropriate locants.
- When two >C=O groups are present, then ending ‘e’ of alkane is retained and the suffix —‘dione’ is added to the name of parent ketone indicating the locants of ketonic carbonyl groups.
- In case of polyfunctional ketones, higher priority group is given lower number. When ketonic carbonyl is a lower priority group it is named as ‘oxo’, preceded by the locant. In alicyclic ketones, carbonyl carbon is numbered as 1.
Trivial and IUPAC names of some ketones :

Question : Draw structures for the : (1) 2-Methylpentanal (2) Hexan-2-one
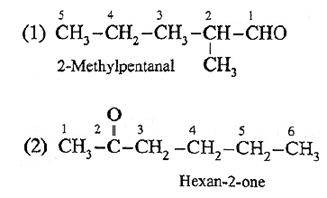
Main Page : – Maharashtra Board Class 12th-Chemistry – All chapters notes, solutions, videos, test, pdf.
Previous Chapter : Chapter 11- Alcohols, Phenols and Ethers – Online Notes
Next Chapter : Chapter-13-Amines– Online Notes
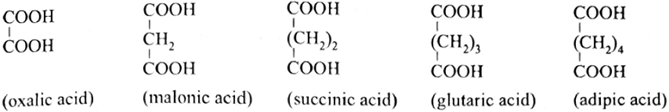
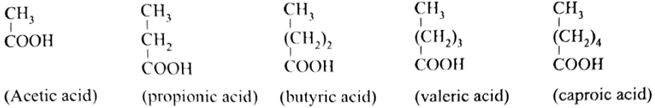
We reply to valid query.